Bimodal endocytic probe for three-dimensional correlative light and electron microscopy
- PMID: 35637912
- PMCID: PMC9142762
- DOI: 10.1016/j.crmeth.2022.100220
Bimodal endocytic probe for three-dimensional correlative light and electron microscopy
Abstract
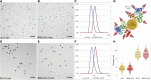
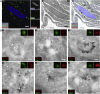
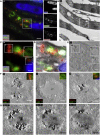
We present a bimodal endocytic tracer, fluorescent BSA-gold (fBSA-Au), as a fiducial marker for 2D and 3D correlative light and electron microscopy (CLEM) applications. fBSA-Au consists of colloidal gold (Au) particles stabilized with fluorescent BSA. The conjugate is efficiently endocytosed and distributed throughout the 3D endolysosomal network of cells and has an excellent visibility in both fluorescence microscopy (FM) and electron microscopy (EM). We demonstrate that fBSA-Au facilitates rapid registration in several 2D and 3D CLEM applications using Tokuyasu cryosections, resin-embedded material, and cryoelectron microscopy (cryo-EM). Endocytosed fBSA-Au benefits from a homogeneous 3D distribution throughout the endosomal system within the cell, does not obscure any cellular ultrastructure, and enables accurate (50-150 nm) correlation of fluorescence to EM data. The broad applicability and visibility in both modalities makes fBSA-Au an excellent endocytic fiducial marker for 2D and 3D (cryo)CLEM applications.
Keywords: correlative light and electron microscopy; cryoelectron microscopy; electron tomography; endolysosomal system; nanogold fiducials; volume-electron microscopy.
© 2022 The Author(s).
Conflict of interest statement
The endocytic fBSA-Au5 and fBSA-Au10 fiducials reported here are available in the product listing of Cell Microscopy Core, UMC Utrecht.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

