High-risk stage IIB Hodgkin lymphoma treated in the H10 and AHL2011 trials: total metabolic tumor volume is a useful risk factor to stratify patients at baseline
- PMID: 35638548
- PMCID: PMC9713544
- DOI: 10.3324/haematol.2021.280004
High-risk stage IIB Hodgkin lymphoma treated in the H10 and AHL2011 trials: total metabolic tumor volume is a useful risk factor to stratify patients at baseline
Abstract
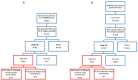
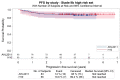
Stage IIB Hodgkin lymphoma (HL) patients, with a mediastinum-to-thorax (M/T) ratio of ≥0.33 or extranodal localization have a poor prognosis and are treated either as limited or advanced stage. We compared these two approaches in patients included in two randomized phase III trials enrolling previously untreated early (H10) or advanced stage HL (AHL2011). We included HL patients with Ann-Arbor stage IIB with M/T ≥0.33 or extranodal involvement enrolled in the H10 or AHL2011 trials with available positron emission tomography at baseline (PET0) and after two cycles of chemotherapy (PET2). Baseline total metabolic tumor volume (TMTV) was calculated using the 41% SUVmax method. PET2 response assessment used the Deauville score. One hundred and fourty-eight patients were eligible, including 83 enrolled in the AHL2011 trial and 65 in the H10 trial. The median TMTV value was 155.5 mL (range, 8.3-782.9 mL), 165.6 mL in AHL2011 and 147 mL in H10. PET2 positivity rates were 16.9% (n=14) and 9.2% (n=6) in AHL2011 and H10 patients, respectively. With a median follow-up of 4.1 years (95% confidence interval [CI]: 3.9-4.4), overall 4-year PFS was 88.0%, 87.0% in AHL2011 and 89.2% in H10. In univariate and mutivariate analyses, baseline TMTV and PET2 response influenced significantly progression-free survival (hazard ratio [HR]=4.94, HR=3.49 respectively). Notably, among the 16 patients who relapsed, 13 (81%) had a baseline TMTV baseline ≥155 mL. Upfront ABVD plus radiation therapy or upfront escBEACOPP without radiotherapy provide similar patient's outcome in high-risk stage IIB HL. TMTV is useful to stratify these patients at baseline.
Figures

References
-
- Driessen J, Visser O, Zijlstra JM, et al. Primary therapy and relative survival in classical Hodgkin lymphoma: a nationwide population-based study in the Netherlands, 1989-2017. Leukemia. 2021;35(2):494-505. - PubMed
-
- Draube A, Behringer K, Diehl V. German Hodgkin’s Lymphoma Study Group Trials: lessons from the past and current strategies. Clin Lymphoma Myeloma. 2006;6(6):458-468. - PubMed
-
- Raemaekers JMM, André MPE, Federico M, et al. Omitting radiotherapy in early positron emission tomography–negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188-1194. - PubMed
-
- André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786-1794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

