HIV and FIV glycoproteins increase cellular tau pathology via cGMP-dependent kinase II activation
- PMID: 35638570
- PMCID: PMC9270957
- DOI: 10.1242/jcs.259764
HIV and FIV glycoproteins increase cellular tau pathology via cGMP-dependent kinase II activation
Abstract
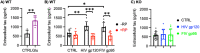
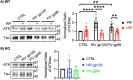
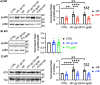
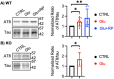
As the development of combination antiretroviral therapy (cART) against human immunodeficiency virus (HIV) drastically improves the lifespan of individuals with HIV, many are now entering the prime age when Alzheimer's disease (AD)-like symptoms begin to manifest. It has been shown that hyperphosphorylated tau, a known AD pathological characteristic, is prematurely increased in the brains of HIV-infected individuals as early as in their 30s and that its levels increase with age. This suggests that HIV infection might lead to accelerated AD phenotypes. However, whether HIV infection causes AD to develop more quickly in the brain is not yet fully determined. Interestingly, we have previously revealed that the viral glycoproteins HIV gp120 and feline immunodeficiency virus (FIV) gp95 induce neuronal hyperexcitation via cGMP-dependent kinase II (cGKII; also known as PRKG2) activation in cultured hippocampal neurons. Here, we use cultured mouse cortical neurons to demonstrate that the presence of HIV gp120 and FIV gp95 are sufficient to increase cellular tau pathology, including intracellular tau hyperphosphorylation and tau release to the extracellular space. We further reveal that viral glycoprotein-induced cellular tau pathology requires cGKII activation. Taken together, HIV infection likely accelerates AD-related tau pathology via cGKII activation.
Keywords: Alzheimer's disease; CGMP-dependent kinase II; Feline immunodeficiency virus; Human immunodeficiency virus; Tau pathology.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Anthony, I. C., Ramage, S. N., Carnie, F. W., Simmonds, P. and Bell, J. E. (2006). Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 111, 529-538. 10.1007/s00401-006-0037-0 - DOI - PubMed
-
- Apetrei, C., Kaur, A., Lerche, N. W., Metzger, M., Pandrea, I., Hardcastle, J., Falkenstein, S., Bohm, R., Koehler, J., Traina-Dorge, V.et al. (2005). Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J. Virol. 79, 8991-9005. 10.1128/JVI.79.14.8991-9005.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

