The Deubiquitinase USP29 Promotes SARS-CoV-2 Virulence by Preventing Proteasome Degradation of ORF9b
- PMID: 35638730
- PMCID: PMC9239186
- DOI: 10.1128/mbio.01300-22
The Deubiquitinase USP29 Promotes SARS-CoV-2 Virulence by Preventing Proteasome Degradation of ORF9b
Abstract
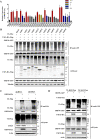
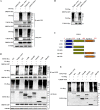
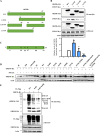
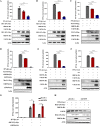
Ubiquitin signaling is essential for immunity to restrict pathogen proliferation. Due to its enormous impact on human health and the global economy, intensive efforts have been invested in studying severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its interactions with hosts. However, the role of the ubiquitin network in pathogenicity has not yet been explored. Here, we found that ORF9b of SARS-CoV-2 is ubiquitinated on Lys-4 and Lys-40 by unknown E3 ubiquitin ligases and is degraded by the ubiquitin proteasomal system. Importantly, we identified USP29 as a host factor that prevents ORF9b ubiquitination and subsequent degradation. USP29 interacts with the carboxyl end of ORF9b and removes ubiquitin chains from the protein, thereby inhibiting type I interferon (IFN) induction and NF-κB activation. We also found that ORF9b stabilization by USP29 enhanced the virulence of VSV-eGFP and transcription and replication-competent SARS-CoV-2 virus-like-particles (trVLP). Moreover, we observed that the mRNA level of USP29 in SARS-CoV-2 patients was higher than that in healthy people. Our findings provide important evidence indicating that targeting USP29 may effectively combat SARS-CoV-2 infection. IMPORTANCE Coronavirus disease 2019 (COVID-19) is a current global health threat caused by SARS-CoV-2. The innate immune response such as type I IFN (IFN-I) is the first line of host defense against viral infections, whereas SARS-CoV-2 proteins antagonize IFN-I production through distinct mechanisms. Among them, ORF9b inhibits the canonical IκB kinase alpha (IKKɑ)/β/γ-NF-κB signaling and subsequent IFN production; therefore, discovering the regulation of ORF9b by the host might help develop a novel antiviral strategy. Posttranslational modification of proteins by ubiquitination regulates many biological processes, including viral infections. Here, we report that ORF9b is ubiquitinated and degraded through the proteasome pathway, whereas deubiquitinase USP29 deubiquitinates ORF9b and prevents its degradation, resulting in the enhancement of ORF9b-mediated inhibition of IFN-I and NF-κB activation and the enhancement of virulence of VSV-eGFP and SARS-CoV-2 trVLP.
Keywords: ORF9b; SARS-CoV-2; USP29; degradation; deubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO.Cell Rep. 2021 Feb 16;34(7):108761. doi: 10.1016/j.celrep.2021.108761. Epub 2021 Feb 3. Cell Rep. 2021. PMID: 33567255 Free PMC article.
-
Multiple E3 ligases act as antiviral factors against SARS-CoV-2 via inducing the ubiquitination and degradation of ORF9b.J Virol. 2024 Jun 13;98(6):e0162423. doi: 10.1128/jvi.01624-23. Epub 2024 May 6. J Virol. 2024. PMID: 38709105 Free PMC article.
-
SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways.J Med Virol. 2021 Sep;93(9):5376-5389. doi: 10.1002/jmv.27050. Epub 2021 May 9. J Med Virol. 2021. PMID: 33913550 Free PMC article.
-
Role of E3 ubiquitin ligases and deubiquitinating enzymes in SARS-CoV-2 infection.Front Cell Infect Microbiol. 2023 Jun 9;13:1217383. doi: 10.3389/fcimb.2023.1217383. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37360529 Free PMC article. Review.
-
SARS-CoV-2 and UPS with potentials for therapeutic interventions.Gene. 2024 Jun 20;912:148377. doi: 10.1016/j.gene.2024.148377. Epub 2024 Mar 14. Gene. 2024. PMID: 38490508 Review.
Cited by
-
Proteome and ubiquitinome analyses of the brain cortex in K18-hACE2 mice infected with SARS-CoV-2.iScience. 2024 Jul 27;27(9):110602. doi: 10.1016/j.isci.2024.110602. eCollection 2024 Sep 20. iScience. 2024. PMID: 39211577 Free PMC article.
-
Cepharanthine analogs mining and genomes of Stephania accelerate anti-coronavirus drug discovery.Nat Commun. 2024 Feb 20;15(1):1537. doi: 10.1038/s41467-024-45690-5. Nat Commun. 2024. PMID: 38378731 Free PMC article.
-
Elevated ubiquitination contributes to protective immunity against severe SARS-CoV-2 infection.Clin Transl Med. 2022 Dec;12(12):e1103. doi: 10.1002/ctm2.1103. Clin Transl Med. 2022. PMID: 36447039 Free PMC article.
-
Exploration of the common genetic landscape of COVID-19 and male infertility.Front Immunol. 2023 Mar 20;14:1123913. doi: 10.3389/fimmu.2023.1123913. eCollection 2023. Front Immunol. 2023. PMID: 37020555 Free PMC article.
-
Deubiquitinase USP1 regulates sarbecovirus ORF6 protein function.J Virol. 2024 Jan 23;98(1):e0143723. doi: 10.1128/jvi.01437-23. Epub 2023 Dec 12. J Virol. 2024. PMID: 38084957 Free PMC article.
References
-
- Han L, Zhuang MW, Deng J, Zheng Y, Zhang J, Nan ML, Zhang XJ, Gao C, Wang PH. 2021. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J Med Virol 93:5376–5389. doi:10.1002/jmv.27050. - DOI - PMC - PubMed
-
- Wu J, Shi Y, Pan X, Wu S, Hou R, Zhang Y, Zhong T, Tang H, Du W, Wang L, Wo J, Mu J, Qiu Y, Yang K, Zhang LK, Ye BC, Qi N. 2021. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep 34:108761. doi:10.1016/j.celrep.2021.108761. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

