Race against Time between the Virus and Host: Actin-Assisted Rapid Biogenesis of Replication Organelles is Used by TBSV to Limit the Recruitment of Cellular Restriction Factors
- PMID: 35638821
- PMCID: PMC9215244
- DOI: 10.1128/jvi.00168-21
Race against Time between the Virus and Host: Actin-Assisted Rapid Biogenesis of Replication Organelles is Used by TBSV to Limit the Recruitment of Cellular Restriction Factors
Abstract
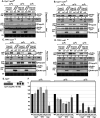
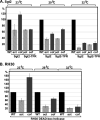
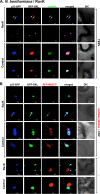
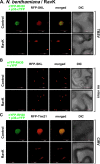
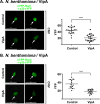
Positive-strand RNA viruses build large viral replication organelles (VROs) with the help of coopted host factors. Previous works on tomato bushy stunt virus (TBSV) showed that the p33 replication protein subverts the actin cytoskeleton by sequestering the actin depolymerization factor, cofilin, to reduce actin filament disassembly and stabilize the actin filaments. Then, TBSV utilizes the stable actin filaments as "trafficking highways" to deliver proviral host factors into the protective VROs. In this work, we show that the cellular intrinsic restriction factors (CIRFs) also use the actin network to reach VROs and inhibit viral replication. Disruption of the actin filaments by expression of the Legionella RavK protease inhibited the recruitment of plant CIRFs, including the CypA-like Roc1 and Roc2 cyclophilins, and the antiviral DDX17-like RH30 DEAD box helicase into VROs. Conversely, temperature-sensitive actin and cofilin mutant yeasts with stabilized actin filaments reduced the levels of copurified CIRFs, including cyclophilins Cpr1, CypA, Cyp40-like Cpr7, cochaperones Sgt2, the Hop-like Sti1, and the RH30 helicase in viral replicase preparations. Dependence of the recruitment of both proviral and antiviral host factors into VROs on the actin network suggests that there is a race going on between TBSV and its host to exploit the actin network and ultimately to gain the upper hand during infection. We propose that, in the highly susceptible plants, tombusviruses efficiently subvert the actin network for rapid delivery of proviral host factors into VROs and ultimately overcome host restriction factors via winning the recruitment race and overwhelming cellular defenses. IMPORTANCE Replication of positive-strand RNA viruses is affected by the recruitment of host components, which provide either proviral or antiviral functions during virus invasion of infected cells. The delivery of these host factors into the viral replication organelles (VROs), which represent the sites of viral RNA replication, depends on the cellular actin network. Using TBSV, we uncover a race between the virus and its host with the actin network as the central player. We find that in susceptible plants, tombusviruses exploit the actin network for rapid delivery of proviral host factors into VROs and ultimately overcome host restriction factors. In summary, this work demonstrates that the actin network plays a major role in determining the outcome of viral infections in plants.
Keywords: DEAD box RNA helicase; cochaperone; cyclophilin; host factor; plant; replication; restriction factor; tomato bushy stunt virus; viral replicase; virus-host interaction; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zorzatto C, Machado JP, Lopes KV, Nascimento KJ, Pereira WA, Brustolini OJ, Reis PA, Calil IP, Deguchi M, Sachetto-Martins G, Gouveia BC, Loriato VA, Silva MA, Silva FF, Santos AA, Chory J, Fontes EP. 2015. NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 520:679–682. 10.1038/nature14171. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

