Peptide Conjugates Derived from flg15, Pep13, and PIP1 That Are Active against Plant-Pathogenic Bacteria and Trigger Plant Defense Responses
- PMID: 35638842
- PMCID: PMC9238401
- DOI: 10.1128/aem.00574-22
Peptide Conjugates Derived from flg15, Pep13, and PIP1 That Are Active against Plant-Pathogenic Bacteria and Trigger Plant Defense Responses
Abstract
Thirty peptide conjugates were designed by combining an antimicrobial peptide (BP16, BP100, BP143, KSL-W, BP387, or BP475) at the N- or C-terminus of a plant defense elicitor peptide (flg15, BP13, Pep13, or PIP1). These conjugates were highly active in vitro against six plant-pathogenic bacteria, especially against Xanthomonas arboricola pv. pruni, Xanthomonas fragariae and Xanthomonas axonopodis pv. vesicatoria. The most active peptides were those incorporating Pep13. The order of the conjugation influenced the antibacterial activity and the hemolysis. Regarding the former, peptide conjugates incorporating the elicitor peptide flg15 or Pep13 at the C-terminus were, in general, more active against Pseudomonas syringae pv. actinidiae and P. syringae pv. syringae, whereas those bearing these elicitor peptides at the N-terminus displayed higher activity against Erwinia. amylovora and the Xanthomonas species. The best peptide conjugates displayed MIC values between 0.8 and 12.5 μM against all the bacteria tested and also had low levels of hemolysis and low phytotoxicity. Analysis of the structural and physicochemical parameters revealed that a positive charge ranging from +5 to +7 and a moderate hydrophobic moment/amphipathic character is required for an optimal biological profile. Interestingly, flg15-BP475 exhibited a dual activity, causing the upregulation of the same genes as flg15 and reducing the severity of bacterial spot in tomato plants with a similar or even higher efficacy than copper oxychloride. Characterization by nuclear magnetic resonance (NMR) of the secondary structure of flg15-BP475 showed that residues 10 to 25 fold into an α-helix. This study establishes trends to design new bifunctional peptides useful against plant diseases caused by plant-pathogenic bacteria. IMPORTANCE The consequences of plant pathogens on crop production together with the lack of effective and environmentally friendly pesticides evidence the need of new agents to control plant diseases. Antimicrobial and plant defense elicitor peptides have emerged as good candidates to tackle this problem. This study focused on combining these two types of peptides into a single conjugate with the aim to potentiate the activity of the individual fragments. Differences in the biological activity of the resulting peptide conjugates were obtained depending on their charge, amphipathicity, and hydrophobicity, as well as on the order of the conjugation of the monomers. This work provided bifunctional peptide conjugates able to inhibit several plant-pathogenic bacteria, to stimulate plant defense responses, and to reduce the severity of bacterial spot in tomato plants. Thus, this study could serve as the basis for the development of new antibacterial/plant defense elicitor peptides to control bacterial plant pathogens.
Keywords: antimicrobial peptide; peptide conjugate; plant defense elicitor; plant disease; secondary structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


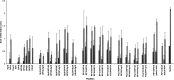
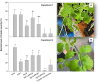

References
-
- Keymanesh K, Soltani S, Sardari S. 2009. Application of antimicrobial peptides in agriculture and food industry. World J Microbiol Biotechnol 25:933–944. 10.1007/s11274-009-9984-7. - DOI
-
- Montesinos E, Badosa E, Cabrefiga J, Planas M, Feliu L, Bardají E. 2012. Antimicrobial peptides for plant disease control. From discovery to application, p 235–262. In Rajasekaran K, Cary JW, Jaynes JM, Montesinos E (ed), Small wonders: peptides for disease control. ACS Symposium Series. American Chemical Society, Washington, DC.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

