Presepsin for the Diagnosis of Neonatal Early-Onset Sepsis: A Systematic Review and Meta-analysis
- PMID: 35639395
- PMCID: PMC9157383
- DOI: 10.1001/jamapediatrics.2022.1647
Presepsin for the Diagnosis of Neonatal Early-Onset Sepsis: A Systematic Review and Meta-analysis
Abstract
Importance: Neonatal early-onset sepsis (EOS) is a severe disease, particularly in preterm infants. Timely diagnosis can be challenging owing to unspecific presentation and questionable performance of the common markers of infection. Presepsin was recently proven to be a promising biomarker for the diagnosis of EOS.
Objective: To assess presepsin accuracy for the diagnosis of EOS.
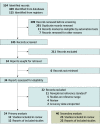
Data sources: PubMed Medline, EMBASE, Web of Science, and Google Scholar. No publication date restrictions were applied. The literature search was limited to the English language. Articles were checked for duplication.
Study selection: Inclusion criteria were studies that (1) included term or preterm newborns (defined as newborns with gestational age ≥37 weeks or <37 weeks, respectively); (2) included a diagnosis of EOS, defined as culture-proven sepsis for primary analysis and as either clinical or culture-proven sepsis for secondary analysis; and (3) assessed presepsin values during the initial workup for suspected EOS. Exclusion criteria were studies that (1) did not include EOS cases; (2) lacked data on presepsin sensitivity and/or specificity; and (3) were case reports, commentaries, or reviews. Two independent reviewers performed the study selection.
Data extraction and synthesis: Two independent reviewers performed data extraction and quality assessment. Quality assessment was performed using the Quality Assessment for Studies of Diagnostic Accuracy 2 tool, and data were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Data were pooled using a random-effects model.
Main outcomes and measures: The outcomes of interest for both the primary and secondary analyses were presepsin sensitivity, specificity, and diagnostic odds ratio for the diagnosis of EOS.
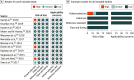
Results: A total of 12 studies of 245 (4.9%) met inclusion criteria for the primary analysis. Twenty-three studies of 245 (9.4%) met the inclusion criteria for the secondary analysis. In the primary analysis, among 12 studies and 828 newborns of any gestational age, pooled sensitivity and specificity were 0.93 (95% CI, 0.86-0.95) and 0.91 (95% CI, 0.85-0.95), respectively; pooled diagnostic odds ratio was 131.69 (95% CI, 54.93-310.94). Subgroup analysis showed that presepsin specificity was associated with the inclusion of only EOS or all neonatal sepsis. Presepsin accuracy was not associated with gestational age, measurement with chemiluminescence enzyme immunoassay or enzyme-linked immunosorbent assay testing, country where the study was performed, or risk of bias judgment. In the secondary analysis, among 23 studies and 1866 newborns, accuracy was significantly associated with only test type.
Conclusions and relevance: Results of this systematic review and meta-analysis suggest that presepsin was an accurate biomarker of EOS. Clinical trials are warranted to assess its usefulness and safety to reduce early antibiotic exposure, particularly in preterm newborns.
Conflict of interest statement
Figures


References
-
- Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi:10.1001/jama.2015.10244 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

