Encoding laboratory testing data: case studies of the national implementation of HHS requirements and related standards in five laboratories
- PMID: 35639494
- PMCID: PMC9277627
- DOI: 10.1093/jamia/ocac072
Encoding laboratory testing data: case studies of the national implementation of HHS requirements and related standards in five laboratories
Abstract
Objective: Assess the effectiveness of providing Logical Observation Identifiers Names and Codes (LOINC®)-to-In Vitro Diagnostic (LIVD) coding specification, required by the United States Department of Health and Human Services for SARS-CoV-2 reporting, in medical center laboratories and utilize findings to inform future United States Food and Drug Administration policy on the use of real-world evidence in regulatory decisions.
Materials and methods: We compared gaps and similarities between diagnostic test manufacturers' recommended LOINC® codes and the LOINC® codes used in medical center laboratories for the same tests.
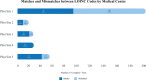
Results: Five medical centers and three test manufacturers extracted data from laboratory information systems (LIS) for prioritized tests of interest. The data submission ranged from 74 to 532 LOINC® codes per site. Three test manufacturers submitted 15 LIVD catalogs representing 26 distinct devices, 6956 tests, and 686 LOINC® codes. We identified mismatches in how medical centers use LOINC® to encode laboratory tests compared to how test manufacturers encode the same laboratory tests. Of 331 tests available in the LIVD files, 136 (41%) were represented by a mismatched LOINC® code by the medical centers (chi-square 45.0, 4 df, P < .0001).
Discussion: The five medical centers and three test manufacturers vary in how they organize, categorize, and store LIS catalog information. This variation impacts data quality and interoperability.
Conclusion: The results of the study indicate that providing the LIVD mappings was not sufficient to support laboratory data interoperability. National implementation of LIVD and further efforts to promote laboratory interoperability will require a more comprehensive effort and continuing evaluation and quality control.
Keywords: Logical Observation Identifiers Names and Codes; clinical laboratory information systems; health information interoperability; public reporting of healthcare data.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures
References
-
- LOINC® from Regenstrief. Home. LOINC®. 2021. http://www.LOINC®.org/. Accessed June 15, 2021.
-
- SNOMED CT® Starter Guide—SNOMED CT® Starter Guide. SNOMED International. 2021. https://confluence.ihtsdotools.org/display/DOCSTART/SNOMED+CT+Starter+Guide. Accessed September 28, 2021.
-
- Paxton A. Checklist, CLIA Line up on COVID Reporting. CAP Today. November 2020. https://www.captodayonline.com/checklist-clia-line-up-on-covid-reporting/. Accessed March 4, 2021.
-
- SHIELD—Standardization of Lab Data to Enhance Patient-Centered Outcomes Research and Value-Based Care. Office of the Assistant Secretary for Planning and Evaluation. https://aspe.hhs.gov/shield-standardization-lab-data-enhance-patient-cen.... Accessed March 4, 2021.
-
- Rubinstein W. CancerLinQ: cutting the Gordian knot of interoperability. J Oncol Pract 2019; 15 (1): 3–6. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U.S. Food and Drug Administration Office of In Vitro Diagnostics and Radiological Health
- # 75F40119C10164/Center for Devices and Radiological Health
- Implementation of SHIELD Harmonized Laboratory Data Standards in Healthcare Institutions
- 75F40119C10164/U.S. Food and Drug Administration Office of In Vitro Diagnostics and Radiological Health within the Center for Devices and Radiological Health
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous