Citicoline in acute ischemic stroke: A randomized controlled trial
- PMID: 35639720
- PMCID: PMC9154187
- DOI: 10.1371/journal.pone.0269224
Citicoline in acute ischemic stroke: A randomized controlled trial
Abstract
Introduction: Two pharmacological possibilities exist for an acute ischemic stroke (AIS): recanalization of the occluded artery and neuroprotection from ischaemic injury, the latter's efficacy being debatable. We sought to determine whether administration of Citicoline immediately after recanalization therapy for AIS would improve clinical and radiological outcome at three months compared to standard treatment alone.
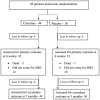
Patients and methods: CAISR was a single centre, randomized, placebo-controlled, parallel-group trial with blinded endpoint assessment. It was approved by the All India Institute of Medical Sciences Institutional ethics committee and registered at the Clinical Trial Registry of India (CTRI/2018/011900). We recruited participants with AIS undergoing recanalization therapy and randomly assigned them to receive either Citicoline or placebo in 1:1 ratio. Citicoline arm patients received Citicoline 1gm BD intravenously for three days, followed by oral citicoline 1gm BD for 39 days. Placebo arm patients received 100ml intravenous normal saline for three days, followed by multivitamin tablet BD for 39 days. All patients received standard of care.
Outcome: Blinded assessors did the follow-up assessment at six weeks (MRI Brain-stroke volume) and three months (NIHSS 0-2, mRS 0-2 and Barthel index> = 95).
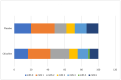
Results: The infarct volume decreased from week 1 to week 6 by 2.6 cm3 on placebo versus 4.2 cm3 on Citicoline (p-0.483). The OR for achieving NIHSS 0-2, mRS 0-2 and Barthel index> = 95 with Citicoline was found to be 0.96(95%CI 0.39-2.40), 0.92(95%CI 0.40-2.05) and 0.87(95%CI 0.22-2.98) respectively.
Conclusion: CAISR was the first to evaluate the role of Citicoline, when used immediately after recanalization therapy, when the penumbral tissue is the most susceptible either to be protected from injury or become ischemic. We did not find any significant difference between the Citicoline or placebo arms with respect to either our primary or secondary outcomes.
Conflict of interest statement
NO authors have competing interests.
Figures
References
-
- Davalos A, Alvarez-Sabin J, Castillo J, et al., for the International Citicoline Trial on Acute Stroke (ICTUS) Trial Investigators. Citicoline in the treatment of acute ischaemic stroke: an international, randomized, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012; 380: 349–357. doi: 10.1016/S0140-6736(12)60813-7 - DOI - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, et al., and the ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375: 1695–703. doi: 10.1016/S0140-6736(10)60491-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical