Mutational analysis of SARS-CoV-2 variants of concern reveals key tradeoffs between receptor affinity and antibody escape
- PMID: 35639784
- PMCID: PMC9223403
- DOI: 10.1371/journal.pcbi.1010160
Mutational analysis of SARS-CoV-2 variants of concern reveals key tradeoffs between receptor affinity and antibody escape
Abstract
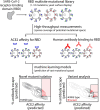
SARS-CoV-2 variants with enhanced transmissibility represent a serious threat to global health. Here we report machine learning models that can predict the impact of receptor-binding domain (RBD) mutations on receptor (ACE2) affinity, which is linked to infectivity, and escape from human serum antibodies, which is linked to viral neutralization. Importantly, the models predict many of the known impacts of RBD mutations in current and former Variants of Concern on receptor affinity and antibody escape as well as novel sets of mutations that strongly modulate both properties. Moreover, these models reveal key opposing impacts of RBD mutations on transmissibility, as many sets of RBD mutations predicted to increase antibody escape are also predicted to reduce receptor affinity and vice versa. These models, when used in concert, capture the complex impacts of SARS-CoV-2 mutations on properties linked to transmissibility and are expected to improve the development of next-generation vaccines and biotherapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

