Functional editing of endogenous genes through rapid selection of cell pools (Rapid generation of endogenously tagged genes in Drosophila ovarian somatic sheath cells)
- PMID: 35639929
- PMCID: PMC9410888
- DOI: 10.1093/nar/gkac448
Functional editing of endogenous genes through rapid selection of cell pools (Rapid generation of endogenously tagged genes in Drosophila ovarian somatic sheath cells)
Abstract
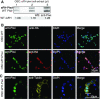
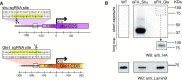
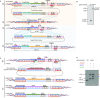
The combination of genome-editing and epitope tagging provides a powerful strategy to study proteins with high affinity and specificity while preserving their physiological expression patterns. However, stably modifying endogenous genes in cells that do not allow for clonal selection has been challenging. Here, we present a simple and fast strategy to generate stable, endogenously tagged alleles in a non-transformed cell culture model. At the example of piwi in Drosophila ovarian somatic sheath cells, we show that this strategy enables the generation of an N-terminally tagged protein that emulates the expression level and subcellular localization of the wild type protein and forms functional Piwi-piRNA complexes. We further present a concise workflow to establish endogenously N-terminally and C-terminally tagged proteins, and knockout alleles through rapid selection of cell pools in fly and human models.
Published by Oxford University Press on behalf of Nucleic Acids Research 2022.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

