Exploring the Influence of Experimental Design on Toxicity Outcomes in Zebrafish Embryo Tests
- PMID: 35639960
- PMCID: PMC9609873
- DOI: 10.1093/toxsci/kfac053
Exploring the Influence of Experimental Design on Toxicity Outcomes in Zebrafish Embryo Tests
Abstract
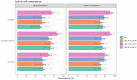
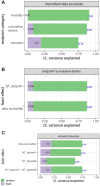
Compound toxicity data obtained from independent zebrafish laboratories can vary vastly, complicating the use of zebrafish screening for regulatory decisions. Differences in the assay protocol parameters are the primary source of variability. We investigated this issue by utilizing data from the NTP DNT-DIVER database (https://doi.org/10.22427/NTP-DATA-002-00062-0001-0000-1, last accessed June 2, 2022), which consists of data from zebrafish developmental toxicity (devtox) and locomotor response (designated as "neurotox") screens from 3 independent laboratories, using the same set of 87 compounds. The data were analyzed using the benchmark concentration (BMC) modeling approach, which estimates the concentration of interest based on a predetermined response threshold. We compared the BMC results from 3 laboratories (A, B, C) in 3 toxicity outcome categories: mortality, cumulative devtox, and neurotox, in terms of activity calls and potency values. We found that for devtox screening, laboratories with similar/same protocol parameters (B vs C) had an active call concordance as high as 86% with negligible potency difference. For neurotox screening, active call concordances between paired laboratories are lower than devtox screening (highest 68%). When protocols with different protocol parameters were compared, the concordance dropped, and the potency shift was on average about 3.8-fold for the cumulative devtox outcome and 5.8-fold for the neurotox outcome. The potential contributing protocol parameters for potency shift are listed or ranked. This study provides a quantitative assessment of the source of variability in zebrafish screening protocols and sets the groundwork for the ongoing Systematic Evaluation of the Application of Zebrafish in Toxicology effort at the National Toxicology Program.
Keywords: computational toxicology; developmental neurotoxicity; developmental toxicity; zebrafish.
Published by Oxford University Press on behalf of the Society of Toxicology 2022.
Figures


References
-
- Ball J. S., Stedman D. B., Hillegass J. M., Zhang C. X., Panzica-Kelly J., Coburn A., Enright B. P., Tornesi B., Amouzadeh H. R., Hetheridge M., et al. (2014). Fishing for teratogens: A consortium effort for a harmonized zebrafish developmental toxicology assay. Toxicol. Sci. 139, 210–219. - PubMed
-
- Beekhuijzen M., de Koning C., Flores-Guillén M.-E., de Vries-Buitenweg S., Tobor-Kaplon M., van de Waart B., Emmen H. (2015). From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 56, 64–76. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

