Schizophrenia in Translation: Why the Eye?
- PMID: 35640030
- PMCID: PMC9212100
- DOI: 10.1093/schbul/sbac050
Schizophrenia in Translation: Why the Eye?
Abstract
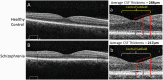
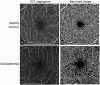
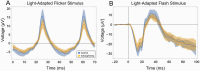
Schizophrenia is increasingly recognized as a systemic disease, characterized by dysregulation in multiple physiological systems (eg, neural, cardiovascular, endocrine). Many of these changes are observed as early as the first psychotic episode, and in people at high risk for the disorder. Expanding the search for biomarkers of schizophrenia beyond genes, blood, and brain may allow for inexpensive, noninvasive, and objective markers of diagnosis, phenotype, treatment response, and prognosis. Several anatomic and physiologic aspects of the eye have shown promise as biomarkers of brain health in a range of neurological disorders, and of heart, kidney, endocrine, and other impairments in other medical conditions. In schizophrenia, thinning and volume loss in retinal neural layers have been observed, and are associated with illness progression, brain volume loss, and cognitive impairment. Retinal microvascular changes have also been observed. Abnormal pupil responses and corneal nerve disintegration are related to aspects of brain function and structure in schizophrenia. In addition, studying the eye can inform about emerging cardiovascular, neuroinflammatory, and metabolic diseases in people with early psychosis, and about the causes of several of the visual changes observed in the disorder. Application of the methods of oculomics, or eye-based biomarkers of non-ophthalmological pathology, to the treatment and study of schizophrenia has the potential to provide tools for patient monitoring and data-driven prediction, as well as for clarifying pathophysiology and course of illness. Given their demonstrated utility in neuropsychiatry, we recommend greater adoption of these tools for schizophrenia research and patient care.
Keywords: ERG; OCT; brain; cornea; eye; imaging; oculomics; pupil; retina; schizophrenia.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Maryland Psychiatric Research Center. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Alonso Y, Valiente-Palleja A, Verge B, Vilella E, Martorell L. High frequency of clinical conditions commonly associated with mitochondrial disorders in schizophrenia. Acta Neuropsychiatr. 2020;32(5):265–269. - PubMed
-
- Little JD. Schizophrenia: a multi-system disorder? Aust N Z J Psychiatry. 2015;49(4):390. - PubMed
-
- Malaspina D. Looking schizophrenia in the eye. Am J Psychiatry. 2013;170(12):1382–1384. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

