A randomized controlled trial comparing perioperative vs. postoperative mFOLFOX6 for lower rectal cancer with suspected lateral pelvic lymph node metastasis (JCOG1310): a phase II/III randomized controlled trial
- PMID: 35640246
- PMCID: PMC9354501
- DOI: 10.1093/jjco/hyac080
A randomized controlled trial comparing perioperative vs. postoperative mFOLFOX6 for lower rectal cancer with suspected lateral pelvic lymph node metastasis (JCOG1310): a phase II/III randomized controlled trial
Erratum in
-
Correction.Jpn J Clin Oncol. 2022 Nov 3;52(11):1358. doi: 10.1093/jjco/hyac156. Jpn J Clin Oncol. 2022. PMID: 36124846 Free PMC article. No abstract available.
Abstract
Objective: The optimal perioperative chemotherapy for lower rectal cancer with lateral pelvic lymph node metastasis remains unclear. We evaluated the efficacy and safety of perioperative mFOLFOX6 in comparison with postoperative mFOLFOX6 for rectal cancer patients undergoing total mesorectal excision with lateral lymph node dissection.
Methods: We conducted an open label randomized phase II/III trial in 18 Japanese institutions. We enrolled patients with histologically proven lower rectal adenocarcinoma with clinical pelvic lateral lymph node metastasis who were randomly assigned (1:1) to receive postoperative mFOLFOX6 (12 courses of intravenous oxaliplatin [85 mg/m2] with L-leucovorin [200 mg/m2] followed by 5-fluorouracil [400 mg/m2, bolus and 2400 mg/m2, continuous infusion, repeated every 2 weeks]) or perioperative mFOLFOX6 (six courses each preoperatively and postoperatively). The primary endpoint was overall survival (OS). The trial is registered with Japan Registry of Clinical Trials, number jRCTs031180230.
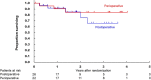
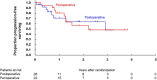
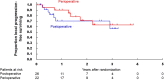
Results: Between May 2015, and May 2019, 48 patients were randomized to the postoperative arm (n = 26) and the perioperative arm (n = 22). The trial was terminated prematurely due to poor accrual. The 3-year OS in the postoperative and perioperative groups were 66.1 and 84.4%, respectively (HR 0.58, 95% CI [0.14-2.45], one-sided P = 0.23). The pathological complete response rate in the perioperative group was 9.1%. Grade 3 postoperative surgical complications were more frequently observed in the perioperative arm (50.0 vs. 12.0%). One treatment-related death due to sepsis from pelvic infection occurred in the postoperative group.
Conclusions: Perioperative mFOLFOX6 may be an insufficient treatment to improve survival of lower rectal cancer with lateral pelvic lymph node metastasis.
Keywords: lateral lymph node dissection; lateral pelvic lymph node metastasis; perioperative chemotherapy; postoperative chemotherapy; rectal cancer.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
-
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–82. - PubMed
-
- Fujita S, Akasu T, Mizusawa J, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol 2012;13:616–21. - PubMed
-
- Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): a multicenter, randomized controlled, noninferiority trial. Ann Surg 2017;266:201–7. - PubMed
-
- Tsukamoto S, Fujita S, Ota M, et al. Long-term follow-up of the randomized trial of mesorectal excision with or without lateral lymph node dissection in rectal cancer (JCOG0212). Br J Surg 2020;107:586–94. - PubMed
-
- Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer. Is it regional or distant disease? Ann Surg 2012;255:1129–34. - PubMed

