Effectiveness of 7-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease in Medically At-Risk Children in Australia: A Record Linkage Study
- PMID: 35640283
- PMCID: PMC9520284
- DOI: 10.1093/jpids/piac038
Effectiveness of 7-Valent Pneumococcal Conjugate Vaccine Against Invasive Pneumococcal Disease in Medically At-Risk Children in Australia: A Record Linkage Study
Abstract
Background: Children with chronic medical conditions are at higher risk of invasive pneumococcal disease (IPD), but little is known about the effectiveness of the primary course of pneumococcal conjugate vaccine (PCV) in these children.
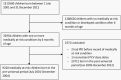
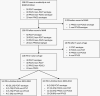
Methods: A cohort born in 2001-2004 from two Australian states and identified as medically at-risk (MAR) of IPD either using ICD-coded hospitalizations (with conditions of interest identified by 6 months of age) or linked perinatal data (for prematurity) were followed to age 5 years for notified IPD by serotype. We categorized fully vaccinated children as either receiving PCV dose 3 by <12 months of age or ≥1 PCV dose at ≥12 months of age. Cox proportional hazard modeling was used to estimate hazard ratios (HRs), adjusted for confounders, and vaccine effectiveness (VE) was estimated as (1-HR) × 100.
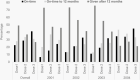
Results: A total of 9220 children with MAR conditions had 53 episodes of IPD (43 vaccine-type); 4457 (48.3%) were unvaccinated and 4246 (46.1%) were fully vaccinated, with 1371 (32.3%) receiving dose 3 by 12 months and 2875 (67.7%) having ≥1 dose at ≥12 months. Estimated VE in fully vaccinated children was 85.9% (95% CI: 33.9-97.0) against vaccine-type IPD and 71.5% (95% CI: 26.6-88.9) against all-cause IPD.
Conclusion: This is the first population-based study evaluating the effectiveness of PCV in children with MAR conditions using record linkage. Our study provides evidence that the VE for vaccine-type and all-cause IPD in MAR children in Australia is high and not statistically different from previously reported estimates for the general population. This method can be replicated in other countries to evaluate VE in MAR children.
Keywords: invasive pneumococcal disease; medically at-risk condition; pneumococcal conjugate vaccine; record linkage; vaccine effectiveness.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Figures
References
-
- Huang SS, Johnson KM, Ray GT, et al. . Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29:3398–412. - PubMed
-
- Toms C. Invasive pneumococcal disease in Australia, 2011 and 2012. Commun Dis Intell Q Rep 2016; 40:E267–84. - PubMed
-
- van Hoek AJ, Andrews N, Waight PA, et al. . The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012; 65:17–24. - PubMed
-
- Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J.. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis 2014; 59:615–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

