Twisting DNA by salt
- PMID: 35640616
- PMCID: PMC9177979
- DOI: 10.1093/nar/gkac445
Twisting DNA by salt
Abstract
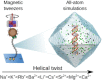
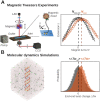
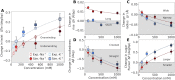
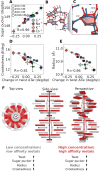
The structure and properties of DNA depend on the environment, in particular the ion atmosphere. Here, we investigate how DNA twist -one of the central properties of DNA- changes with concentration and identity of the surrounding ions. To resolve how cations influence the twist, we combine single-molecule magnetic tweezer experiments and extensive all-atom molecular dynamics simulations. Two interconnected trends are observed for monovalent alkali and divalent alkaline earth cations. First, DNA twist increases monotonously with increasing concentration for all ions investigated. Second, for a given salt concentration, DNA twist strongly depends on cation identity. At 100 mM concentration, DNA twist increases as Na+ < K+ < Rb+ < Ba2+ < Li+ ≈ Cs+ < Sr2+ < Mg2+ < Ca2+. Our molecular dynamics simulations reveal that preferential binding of the cations to the DNA backbone or the nucleobases has opposing effects on DNA twist and provides the microscopic explanation of the observed ion specificity. However, the simulations also reveal shortcomings of existing force field parameters for Cs+ and Sr2+. The comprehensive view gained from our combined approach provides a foundation for understanding and predicting cation-induced structural changes both in nature and in DNA nanotechnology.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Friedberg E.C. DNA damage and repair. Nature. 2003; 421:436–440. - PubMed
-
- Bloomfield V.A. DNA condensation. Curr. Opin. Struct. Biol. 1996; 6:334–341. - PubMed
-
- Bustamante C., Bryant Z., Smith S.B.. Ten years of tension: single-molecule DNA mechanics. Nature. 2003; 421:423–427. - PubMed
-
- Lipfert J., van Oene M.M., Lee M., Pedaci F., Dekker N.H.. Torque spectroscopy for the study of rotary motion in biological systems. Chem. Rev. 2015; 115:1449–1474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

