Noninvasive Ventilation Exposure Prior to Intubation in Pediatric Hematopoietic Cell Transplant Recipients
- PMID: 35640999
- PMCID: PMC9994337
- DOI: 10.4187/respcare.09776
Noninvasive Ventilation Exposure Prior to Intubation in Pediatric Hematopoietic Cell Transplant Recipients
Abstract
Background: Noninvasive ventilation (NIV) has become more studied in immunocompromised patients. However, it has not been studied in hematopoietic cell transplantation (HCT) recipients, who have higher mortality and higher pulmonary complication rates than other immunocompromised patients. This population may be prone to negative effects from this treatment modality. The aim of this study was to determine whether NIV use is associated with worse outcomes in this vulnerable patient population.
Methods: A secondary analysis of a retrospective multi-center database was performed. Twelve pediatric ICUs across the United States enrolled HCT subjects from 2009-2014 that were admitted to the pediatric ICU (PICU) with the diagnosis of acute respiratory failure. Subjects exposed to NIV prior to intubation were compared against those not exposed to NIV. Our primary outcome was all-cause mortality at 90 d; secondary outcomes included ventilator-free days (VFD) at 28 d and development of pediatric ARDS. Multivariable logistic and linear regression models were constructed using variables significant on univariable analysis.
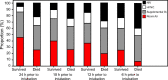
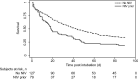
Results: Two-hundred eleven subjects were included. Of these, 82 (39%) received NIV prior to intubation. Those that received NIV prior to intubation were older (13 vs 6 y, P < .001) and more commonly diagnosed with respiratory distress (90% vs 74%, P = .004). On multivariable analysis, NIV use prior to intubation was associated with a higher PICU mortality (hazard ratio 1.51 [95% CI 1.18-2.28], P = .02) and fewer VFD at 28 d (β -3.50 [95% CI -6.09 to 0.91], P = .008). Those with NIV exposure prior to intubation also had higher rates of development of pediatric ARDS (95% vs 78%, P = .001).
Conclusions: In this cohort of children post-HCT, NIV use prior to intubation was associated with worse outcomes. The benefits and risks of NIV in this patient population should be carefully evaluated prior to its use, and careful patient selection is crucial for its optimal utilization.
Keywords: artificial respiration; critical care; hematopoietic stem cell transplantation; mortality; noninvasive ventilation; pediatrics.
Copyright © 2022 by Daedalus Enterprises.
Conflict of interest statement
Dr Rowan is funded by the NIH (1K23HL150244-01A21) The remaining authors have disclosed no conflicts of interest.
Figures
References
-
- Eikenberry M, Bartakova H, Defor T, Haddad IY, Ramsay NK, Blazar BR, et al. . Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant 2005;11(1):56-64. - PubMed
-
- Ciki K, Dogru D, Kuskonmaz B, Emiralioglu N, Yalcin E, Ozcelik U, et al. . Pulmonary complications following hematopoietic stem cell transplantation in children. Turk J Pediatr 2019;61(1):59-60. - PubMed
-
- Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant 2009;15(7):817-826. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

