SARS-CoV-2 induces barrier damage and inflammatory responses in the human iPSC-derived intestinal epithelium
- PMID: 35641026
- PMCID: PMC9060709
- DOI: 10.1016/j.jphs.2022.04.010
SARS-CoV-2 induces barrier damage and inflammatory responses in the human iPSC-derived intestinal epithelium
Abstract
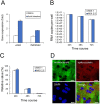
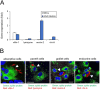
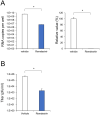
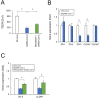
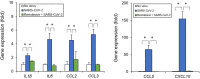
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread and led to global health crises. COVID-19 causes well-known respiratory failure and gastrointestinal symptoms, such as diarrhea, nausea, and vomiting. Thus, human gastrointestinal cell models are urgently needed for COVID-19 research; however, it is difficult to obtain primary human intestinal cells. In this study, we examined whether human induced pluripotent stem cell (iPSC)-derived small intestinal epithelial cells (iPSC-SIECs) could be used as a SARS-CoV-2 infection model. We observed that iPSC-SIECs, such as absorptive and Paneth cells, were infected with SARS-CoV-2, and remdesivir treatment decreased intracellular SARS-CoV-2 replication in iPSC-SIECs. SARS-CoV-2 infection decreased expression levels of tight junction markers, ZO-3 and CLDN1, and transepithelial electrical resistance (TEER), which evaluates the integrity of tight junction dynamics. In addition, SARS-CoV-2 infection increased expression levels of proinflammatory genes, which are elevated in patients with COVID-19. These findings suggest iPSC-SIECs as a useful in vitro model for elucidating COVID-19 pathology and drug development.
Keywords: Barrier functions; COVID-19; SARS-CoV-2; Small intestinal epithelial cells; iPSC.
Copyright © 2022 The Authors. Production and hosting by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
SARS-CoV-2 causes dysfunction in human iPSC-derived brain microvascular endothelial cells potentially by modulating the Wnt signaling pathway.Fluids Barriers CNS. 2024 Apr 8;21(1):32. doi: 10.1186/s12987-024-00533-9. Fluids Barriers CNS. 2024. PMID: 38584257 Free PMC article.
-
Human airway lineages derived from pluripotent stem cells reveal the epithelial responses to SARS-CoV-2 infection.Am J Physiol Lung Cell Mol Physiol. 2022 Mar 1;322(3):L462-L478. doi: 10.1152/ajplung.00397.2021. Epub 2022 Jan 12. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35020534 Free PMC article.
-
Human Pluripotent Stem Cell-Derived Intestinal Organoids Model SARS-CoV-2 Infection Revealing a Common Epithelial Inflammatory Response.Stem Cell Reports. 2021 Apr 13;16(4):940-953. doi: 10.1016/j.stemcr.2021.02.019. Stem Cell Reports. 2021. PMID: 33852884 Free PMC article.
-
Induced pluripotent stem cell-based disease modeling and prospective immune therapy for coronavirus disease 2019.Cytotherapy. 2022 Mar;24(3):235-248. doi: 10.1016/j.jcyt.2021.08.003. Epub 2021 Sep 14. Cytotherapy. 2022. PMID: 34656419 Free PMC article. Review.
-
Pathogenesis and Mechanism of Gastrointestinal Infection With COVID-19.Front Immunol. 2021 Nov 10;12:674074. doi: 10.3389/fimmu.2021.674074. eCollection 2021. Front Immunol. 2021. PMID: 34858386 Free PMC article. Review.
Cited by
-
Regulation of Paneth cell-specific genes in COVID-19 patients and SARS-CoV-2-infected mice by quantification of mRNA from exfoliated cells in stool samples.Sci Rep. 2024 Dec 30;14(1):31740. doi: 10.1038/s41598-024-82098-z. Sci Rep. 2024. PMID: 39738327 Free PMC article.
-
Impairment of Esophageal Barrier Integrity: New Insights into Esophageal Symptoms in Post-COVID-19.Dig Dis Sci. 2025 May 2. doi: 10.1007/s10620-025-09062-3. Online ahead of print. Dig Dis Sci. 2025. PMID: 40316885
-
Organoids to Remodel SARS-CoV-2 Research: Updates, Limitations and Perspectives.Aging Dis. 2023 Oct 1;14(5):1677-1699. doi: 10.14336/AD.2023.0209. Aging Dis. 2023. PMID: 37196111 Free PMC article. Review.
-
Can Lactoferrin, a Natural Mammalian Milk Protein, Assist in the Battle against COVID-19?Nutrients. 2022 Dec 10;14(24):5274. doi: 10.3390/nu14245274. Nutrients. 2022. PMID: 36558432 Free PMC article. Review.
-
Preferential apical infection of Caco-2 intestinal cell monolayers by SARS-CoV-2 is associated with damage to cellular barrier integrity: Implications for the pathophysiology of COVID-19.PLoS One. 2025 Feb 10;20(2):e0313068. doi: 10.1371/journal.pone.0313068. eCollection 2025. PLoS One. 2025. PMID: 39928619 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

