Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti-TNFα therapy: a placebo-controlled phase II study
- PMID: 35641124
- PMCID: PMC9279835
- DOI: 10.1136/annrheumdis-2022-222228
Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti-TNFα therapy: a placebo-controlled phase II study
Abstract
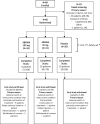
Objective: This phase 2a randomised, double blind, placebo controlled, parallel group study evaluated the safety and efficacy of a first-in-class drug candidate ABX464 (obefazimod, 50 mg and 100 mg per day), which upregulates the biogenesis of the mRNA inhibitor micro-RNA (miR)-124, in combination with methotrexate (MTX) in 60 patients (1:1:1 ratio) with moderate-to-severe active rheumatoid arthritis (RA) who have inadequate response to MTX or/and to an anti-tumour necrosis factor alpha (TNFα) therapy.
Methods: The primary end point was the safety of ABX464; efficacy endpoints included the proportion of patients achieving American College of Rheumatology (ACR)20/50/70 responses, disease activity scores (DAS) 28, simplified disease activity score, clinical disease activity score), European League Against Rheumatism response, DAS28 low disease activity or remission.
Results: ABX464 50 mg was safe and well tolerated. Two serious adverse events were reported (one on placebo group and one on ABX464 100 mg). Eleven patients were withdrawn for AEs (9 patients on 100 mg, 1 on 50 mg and 1 on placebo). Drug discontinuation was mainly due to gastrointestinal disorders. No cases of opportunistic infection, no malignancies and no death were reported. Compared with placebo, ABX464 50 mg showed significantly higher proportions of patients achieving ACR20 and ACR50 responses at week 12. DAS28-C reactive protein (CRP) and DAS28-erythrocyte sedimentation rate decreased significantly and rates of categorical DAS28-CRP response or CDAI remission increased significantly on ABX464 at week 12. A significant upregulation of miR-124 was observed in blood for every patient dosed with ABX464.
Conclusion: ABX464 50 mg was safe, well tolerated and showed a promising efficacy. Mild-to-moderate gastrointestinal AEs led to a high drop-out rate of patients on ABX464 100 mg, which may not be a relevant dose to use. These findings warrant exploration of ABX464 at 50 mg per day or less for treating patients with RA.
Trial registration name: Phase IIa randomised, double blind, placebo controlled, parallel group, multiple dose study on ABX464 in combination with MTX, in patients with moderate to severe active RA who have inadequate response to MTX or/and to an anti- TNFα therapy or intolerance to anti-TNFα therapy.EUDRACT number: 2018-004677-27 TRIAL REGISTRATION NUMBER: NCT03813199.
Keywords: antirheumatic agents; arthritis, rheumatoid; methotrexate.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CD received consulting fees from ABIVAX as PI of the study, and declares consulting fees, punctual links or research grant from Abbvie, Amgen, BMS, Fresenius-Kabi, MSD, Novartis, Pfizer, Sandoz, Sanofi, Roche-Chugai, UCB. PD and MK were investigators contracted by the Sponsor for this study and have nothing else to disclose. LDdR, PG, HE, SB, DS and JS are employees at Abivax. JMS was a former employee at Abivax.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

