Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: the pragmatic randomised, double-blind placebo-controlled GLORIA trial
- PMID: 35641125
- PMCID: PMC9209692
- DOI: 10.1136/annrheumdis-2021-221957
Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: the pragmatic randomised, double-blind placebo-controlled GLORIA trial
Abstract
Background: Low-dose glucocorticoid (GC) therapy is widely used in rheumatoid arthritis (RA) but the balance of benefit and harm is still unclear.
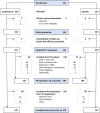
Methods: The GLORIA (Glucocorticoid LOw-dose in RheumatoId Arthritis) pragmatic double-blind randomised trial compared 2 years of prednisolone, 5 mg/day, to placebo in patients aged 65+ with active RA. We allowed all cotreatments except long-term open label GC and minimised exclusion criteria, tailored to seniors. Benefit outcomes included disease activity (disease activity score; DAS28, coprimary) and joint damage (Sharp/van der Heijde, secondary). The other coprimary outcome was harm, expressed as the proportion of patients with ≥1 adverse event (AE) of special interest. Such events comprised serious events, GC-specific events and those causing study discontinuation. Longitudinal models analysed the data, with one-sided testing and 95% confidence limits (95% CL).
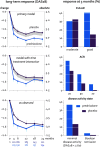
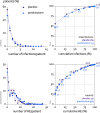
Results: We randomised 451 patients with established RA and mean 2.1 comorbidities, age 72, disease duration 11 years and DAS28 4.5. 79% were on disease-modifying treatment, including 14% on biologics. 63% prednisolone versus 61% placebo patients completed the trial. Discontinuations were for AE (both, 14%), active disease (3 vs 4%) and for other (including covid pandemic-related disease) reasons (19 vs 21%); mean time in study was 19 months. Disease activity was 0.37 points lower on prednisolone (95% CL 0.23, p<0.0001); joint damage progression was 1.7 points lower (95% CL 0.7, p=0.003). 60% versus 49% of patients experienced the harm outcome, adjusted relative risk 1.24 (95% CL 1.04, p=0.02), with the largest contrast in (mostly non-severe) infections. Other GC-specific events were rare.
Conclusion: Add-on low-dose prednisolone has beneficial long-term effects in senior patients with established RA, with a trade-off of 24% increase in patients with mostly non-severe AE; this suggests a favourable balance of benefit and harm.
Trial registration number: NCT02585258.
Keywords: arthritis, rheumatoid; glucocorticoids; osteoporosis; therapeutics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Maarten Boers: Novartis. Linda Hartman: none. Daniela Opris-Belinski: Abbvie, Pfizer, MSD, Novartis, Eli Lilly, Ewo Pharma, UCB. Reinhard Bos: none. Marc R. Kok: none. José A.P. da Silva: none. Eduard N. Griep: none. Ruth Klaasen: none. Cornelia F. Allaart: none. Paul Baudoin: none. Hennie G. Raterman: AbbVie, Amgen, Celgene, Roche, Sandoz, Sanofi Genzyme, UCB. Zoltan Szekanecz: none. Frank Buttgereit: Abbvie, AstraZeneca, Gruenenthal, Horizon Therapeutics, Mundipharma, Pfizer, Roche. Pavol Masaryk: none. L. Thomas Klausch: none. Sabrina Paolino: none. Annemarie M. Schilder: Eli Lilly, Novartis, Genzyme. Willem F. Lems: Pfizer, Galapagos, Lilly, Amgen, UCB. Maurizio Cutolo: none.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

