Allogeneic Cell Combination Therapy Ameliorates Chronic Kidney Disease-Induced Heart Failure with Preserved Ejection Fraction
- PMID: 35641169
- PMCID: PMC8895493
- DOI: 10.1093/stcltm/szab004
Allogeneic Cell Combination Therapy Ameliorates Chronic Kidney Disease-Induced Heart Failure with Preserved Ejection Fraction
Abstract
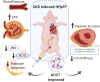
Background: Left ventricular hypertrophy and heart failure with preserved ejection fraction (HFpEF) are primary manifestations of the cardiorenal syndrome in patients with chronic kidney disease (CKD). Therapies that improve morbidity and mortality in HFpEF are lacking. Cell-based therapies promote cardiac repair in ischemic and non-ischemic cardiomyopathies. We hypothesized that cell-based therapy ameliorates CKD-induced HFpEF.
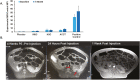
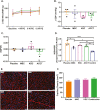
Methods and results: Yorkshire pigs (n = 26) underwent 5/6 embolization-mediated nephrectomy. CKD was confirmed by increased creatinine and decreased glomerular filtration rate (GFR). Mean arterial pressure (MAP) was not different between groups from baseline to 4 weeks. HFpEF was evident at 4 weeks by increased LV mass, relative wall thickening, end-diastolic pressure, and end-diastolic pressure-volume relationship, with no change in ejection fraction (EF). Four weeks post-embolization, allogeneic (allo) bone marrow-derived mesenchymal stem cells (MSC; 1 × 107 cells), allo-kidney-derived stem cells (KSC; 1 × 107 cells), allo-cell combination therapy (ACCT; MSC + KSC; 1:1 ratio; total = 1 × 107 cells), or placebo (Plasma-Lyte) was delivered via intra-renal artery. Eight weeks post-treatment, there was a significant increase in MAP in the placebo group (21.89 ± 6.05 mmHg) compared to the ACCT group. GFR significantly improved in the ACCT group. EF, relative wall thickness, and LV mass did not differ between groups at 12 weeks. EDPVR improved in the ACCT group, indicating decreased ventricular stiffness.
Conclusions: Intra-renal artery allogeneic cell therapy was safe in a CKD swine model manifesting the characteristics of HFpEF. The beneficial effect on renal function and ventricular compliance in the ACCT group supports further research of cell therapy for cardiorenal syndrome.
Keywords: allogeneic cell combination therapy; cardiorenal; cell-based therapy; chronic kidney disease (CKD); heart failure with preserved ejection fraction (HFpEF); kidney-derived stem cells (KSCs); mesenchymal stem cells (MSCs).
© The Author(s) 2022. Published by Oxford University Press.
Figures




References
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM.. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259. - PubMed
-
- Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. - PubMed
-
- Dunlay SM, Roger VL, Redfield MM.. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591-602. - PubMed
-
- Silverman DN, Plante TB, Infeld M, et al. . Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019;2(12):e1916598. - PMC - PubMed
-
- Kjeldsen SE, von Lueder TG, Smiseth OA, et al. . Medical therapies for heart failure with preserved ejection fraction. Hypertension. 2020;75(1):23-32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

