Autophagy modulation in multiple sclerosis and experimental autoimmune encephalomyelitis
- PMID: 35641229
- PMCID: PMC9390842
- DOI: 10.1093/cei/uxac017
Autophagy modulation in multiple sclerosis and experimental autoimmune encephalomyelitis
Abstract
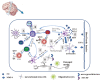
Multiple sclerosis (MS), a white matter demyelinating disease of the central nervous system (CNS), is characterized by neuroinflammatory and neurodegenerative. Experimental autoimmune encephalomyelitis (EAE) is a commonly used animal model for investigating pathogenic mechanisms of MS, representing the destruction of the blood-brain barrier (BBB), the activation of T cells, and the infiltration of myeloid cells. An increasing number of studies have documented that autophagy plays a critical role in the pathogenesis of both MS and EAE. Autophagy maintains CNS homeostasis by degrading the damaged organelles and abnormal proteins. Furthermore, autophagy is involved in inflammatory responses by regulating the activation of immune cells and the secretion of inflammatory factors. However, the specific mechanisms of autophagy involved in MS and EAE are not completely understood. In this review, we will summarize the complex mechanisms of autophagy in MS and EAE, providing potential therapeutic approaches for the management of MS.
Keywords: autophagy; experimental autoimmune encephalomyelitis; immune cells; inflammatory factors; multiple sclerosis.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Immunology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

