Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review
- PMID: 35641489
- PMCID: PMC9152308
- DOI: 10.1038/s41408-022-00684-8
Effectiveness, immunogenicity, and safety of COVID-19 vaccines for individuals with hematological malignancies: a systematic review
Abstract
The efficacy of SARS-CoV-2 vaccination in patients with hematological malignancies (HM) appears limited due to disease and treatment-associated immune impairment. We conducted a systematic review of prospective studies published from 10/12/2021 onwards in medical databases to assess clinical efficacy parameters, humoral and cellular immunogenicity and adverse events (AE) following two doses of COVID-19 approved vaccines. In 57 eligible studies reporting 7393 patients, clinical outcomes were rarely reported and rates of SARS-CoV-2 infection (range 0-11.9%), symptomatic disease (0-2.7%), hospital admission (0-2.8%), or death (0-0.5%) were low. Seroconversion rates ranged from 38.1-99.1% across studies with the highest response rate in myeloproliferative diseases and the lowest in patients with chronic lymphocytic leukemia. Patients with B-cell depleting treatment had lower seroconversion rates as compared to other targeted treatments or chemotherapy. The vaccine-induced T-cell response was rarely and heterogeneously reported (26.5-85.9%). Similarly, AEs were rarely reported (0-50.9% ≥1 AE, 0-7.5% ≥1 serious AE). In conclusion, HM patients present impaired humoral and cellular immune response to COVID-19 vaccination with disease and treatment specific response patterns. In light of the ongoing pandemic with the easing of mitigation strategies, new approaches to avert severe infection are urgently needed for this vulnerable patient population that responds poorly to current COVID-19 vaccine regimens.
© 2022. The Author(s).
Conflict of interest statement
VP, AB, CH, CI, AA, IM, NK, JS, PJB, and NS declare no financial conflicts of interest with regard to the present study. SCM reports grants from DZIF. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, Shionogi; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7); Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley.
Figures
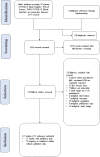


References
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard: World Health Organisation. 2020b. https://covid19.who.int/. Accessed 14 March 2022.
-
- Bertuzzi AF, Ciccarelli M, Marrari A, Gennaro N, Dipasquale A, Giordano L, et al. Impact of active cancer on COVID-19 survival: a matched-analysis on 557 consecutive patients at an Academic Hospital in Lombardy, Italy. Br J Cancer. 2021;125:358–65.. doi: 10.1038/s41416-021-01396-9. - DOI - PMC - PubMed
-
- Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol [Internet]. 2021. http://europepmc.org/abstract/MED/34649563. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

