Immune checkpoint inhibitors alone vs immune checkpoint inhibitors-combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis
- PMID: 35641819
- PMCID: PMC9427994
- DOI: 10.1038/s41416-022-01832-4
Immune checkpoint inhibitors alone vs immune checkpoint inhibitors-combined chemotherapy for NSCLC patients with high PD-L1 expression: a network meta-analysis
Abstract
Background: We indirectly compared the effects of immune checkpoint inhibitors alone (ICI) and ICI-combined chemotherapy (chemo-ICI) in patients with non-small cell lung cancer who had high programmed death-ligand 1 (PD-L1) expression (defined as tumour proportion score ≥50% or TC3/IC3) through network meta-analyses.
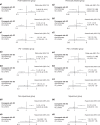
Methods: Through literature searches, we shortlisted 22 randomised controlled trials encompassing 4289 patients, with objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) set as the primary outcomes. The dichotomous data for ORR and hazard ratios (HRs) and their 95% confidence intervals (CIs) for OS and PFS were extracted.
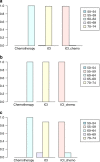
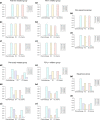
Results: We found that chemo-ICI had significantly improved ORR (OR 1.7, 95% CI 1.1-2.5) and PFS (HR 0.59, 95% CI: 0.48-0.74) relative to ICI. Although no significant difference in OS was observed, the analyses revealed that the chemo-ICI patients tended to undergo fewer progression events than ICI patients (HR 0.82, 95% CI 0.6-1.1). In subgroup analysis, the non-squamous, PD-1 inhibitor and first-line treatment cohorts exhibited significant differences in ORR and PFS, but not in OS. However, in the squamous, PD-L1 inhibitor, and previously treated cohorts, PFS, OS and ORR were not different between chemo-ICI and ICI patients.
Conclusions: In conclusion, for non-squamous NSCLC patients, accepting PD-1 as the first-line treatment may be a relatively better option.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Shi Y, Chen W, Li C, Zhang Y, Bo M, Qi S, et al. Efficacy and safety of first-line treatments with immune checkpoint inhibitors plus chemotherapy for non-squamous non-small cell lung cancer: a meta-analysis and indirect comparison. Ann Palliat Med. 2021;10:2766–75. doi: 10.21037/apm-20-1498. - DOI - PubMed
-
- Pathak R, De Lima LG, Yu H, Aryal MR, Ji W, Frumento KS, et al. Comparative efficacy of chemoimmunotherapy versus immunotherapy for advanced non-small cell lung cancer: a network meta-analysis of randomized trials. Cancer-Am Cancer Soc. 2021;127:709–19. - PubMed
-
- Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto PH, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–69. doi: 10.1016/j.jtho.2020.06.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

