Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review
- PMID: 35641955
- PMCID: PMC9153861
- DOI: 10.1186/s12889-022-13342-2
Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review
Abstract
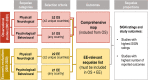
Background: Invasive meningococcal disease (IMD) is uncommon, life-threatening, with many diverse sequelae. The aims were to: 1) comprehensively characterise the sequelae; 2) have a systematic application for sequelae impact in economic evaluation (EE).
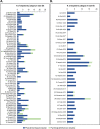
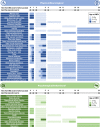
Methods: Sequelae categorised as physical/neurological or psychological/behavioural were identified from a systematic review of IMD observational studies (OS) and EEs in high-income countries (published 2001-2020). A comprehensive map and EE-relevant list, respectively, included physical/neurological sequelae reported in ≥2OS and ≥ 2OS + 2EE (≥1OS and ≥ 1OS + 1EE for psychological/behavioural). Sequelae proportions were selected from the highest quality studies reporting most sequelae. Three medical experts independently evaluated the clinical impact of findings.
Results: Sixty-Six OS and 34 EE reported IMD sequelae. The comprehensive map included 44 sequelae (30 physical/neurological, 14 psychological/behavioural), of which 18 (14 physical/neurological and 4 psychological/behavioural) were EE-relevant. Experts validated the study and identified gaps due to limited evidence, underreporting of psychological/behavioural sequelae in survivors/their families, and occurrence of multiple sequelae in the acute phase and long-term.
Conclusions: The considerable burden of IMD sequelae on survivors and their families is potentially underestimated in EE, due to underreporting and poorly-defined subtle sequelae. When assessing IMD burden and potential interventions e.g., vaccination, sequelae range and duration, underreporting, and indirect burden on dependents should be considered.
Keywords: Economic evaluation; Meningococcal infection; Sequelae; Systematic review.
© 2022. The Author(s).
Conflict of interest statement
KM, YRG and RBB are employees in the GSK group of companies. KM, RBB hold shares in the GSK group of companies. JS was employee and hold share in the GSK group of companies during the conduct of the study. NB is a freelance consultant for the GSK group of companies and therefore received consulting fees during the conduct of the study. FMT reports personal fees, non-financial support and/or grants from Ablynx, Jansen, GSK, Regeneron, Medimmune, Pfizer, MSD Sanofi Pasteur, Novavax, Astra Zeneca, Novartis, Seqirus, Roche, Abott, Instituto de Salud Carlos III and Biofabri, outside the submitted work. JS, NB, YRG, FMT, RBB and KM declare no other financial and non-financial relationships and activities.
Figures

References
-
- National Organization for Rare Disorders (NORD). Meningococcal Meningitis. https://rarediseases.org/rare-diseases/meningococcal-meningitis/. Accessed 30 June 2021.
-
- Centers for Disease Control and Prevention (CDC). Meningococcal Disease - Surveillance 2019. https://www.cdc.gov/meningococcal/surveillance/index.html. Accessed 30 June2021.
-
- European Centre for Disease Prevention and Control (ECDC). Factsheet about meningococcal disease 2019. https://www.ecdc.europa.eu/en/meningococcal-disease/factsheet. Accessed 30 June 2021.
-
- European Centre for Disease Prevention and Control (ECDC). Surveillance report. Invasive meningococcal disease - Annual epidemiological report for 2017. 2017. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-in.... Accessed 27 July 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

