Transcriptomic datasets of cancer patients treated with immune-checkpoint inhibitors: a systematic review
- PMID: 35641998
- PMCID: PMC9153191
- DOI: 10.1186/s12967-022-03409-4
Transcriptomic datasets of cancer patients treated with immune-checkpoint inhibitors: a systematic review
Abstract
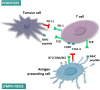
The availability of immune-checkpoint inhibitors (ICI) in the last decade has resulted in a paradigm shift in certain areas of oncology. Patients can be treated either by a monotherapy of anti-CTLA-4 (tremelimumab or ipilimumab), anti-PD-1 (nivolumab or pembrolizumab), or anti-PD-L1 (avelumab or atezolizumab or durvalumab) or as combination therapy of anti-CTLA-4 and anti-PD-1. To maximize the clinical treatment benefit of cancer immunotherapy, the prediction of the actual immune response by the identification and application of clinically useful biomarkers will be required. Whole transcriptomic datasets of patients with ICI treatment could provide the basis for large-scale discovery and ranking of such potential biomarker candidates. In this review, we summarize currently available transcriptomic data from different biological sources (whole blood, fresh-frozen tissue, FFPE) obtained by different methods (microarray, RNA-Seq, RT-qPCR). We directly include only results from clinical trials and other investigations where an ICI treatment was administered. The available datasets are grouped based on the administered treatment and we also summarize the most important results in the individual cohorts. We discuss the limitations and shortcomings of the available datasets. Finally, a subset of animal studies is reviewed to provide an overview of potential in vivo ICI investigations. Our review can provide a swift reference for researchers aiming to find the most suitable study for their investigation, thus saving a significant amount of time.
Keywords: CTLA-4; Clinical data; Gene expression; PD-1; PD-L1; Response; Survival.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. - PubMed
-
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. - PubMed
-
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials