Ethacrynic acid is an inhibitor of human factor XIIIa
- PMID: 35642005
- PMCID: PMC9158266
- DOI: 10.1186/s40360-022-00575-5
Ethacrynic acid is an inhibitor of human factor XIIIa
Abstract
Background: Ethacrynic acid (EA) is a loop diuretic that is approved orally and parenterally to manage edema-associated diseases. Nevertheless, it was earlier reported that it is also associated with bleeding upon its parenteral administration. In this report, we investigated the effects of EA on human factor XIIIa (FXIIIa) of the coagulation process using a variety of techniques.
Methods: A series of biochemical and computational methods have been used in this study. The potency and efficacy of human FXIIIa inhibition by EA was evaluated using a bisubstrate-based fluorescence trans-glutamination assay under near physiological conditions. To establish the physiological relevance of FXIIIa inhibition by EA, the effect on FXIIIa-mediated polymerization of fibrin(ogen) as well as the formation of fibrin(ogen) - α2-antiplasmin complex was evaluated using SDS-PAGE experiments. The selectivity profile of EA against other coagulation proteins was assessed by evaluating EA's effect on human clotting times in the activated partial thromboplastin time (APTT) and the prothrombin time (PT) assays. We also used molecular modeling studies to put forward a putative binding mode for EA in the active site of FXIIIa. Results involving EA were the average of at least three experiments and the standard error ± 1 was provided. In determining the inhibition parameters, we used non-linear regression analysis.
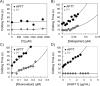
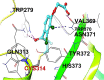
Results: FXIIIa is a transglutaminase that works at the end of the coagulation process to form an insoluble, rigid, and cross-linked fibrin rich blood clot. In fact, inhibition of FXIIIa-mediated biological processes has been reported to result in a bleeding diathesis. Inhibition of FXIIIa by EA was investigated given the nucleophilic nature of the thiol-containing active site of the enzyme and the Michael acceptor-based electrophilicity of EA. In a bisubstrate-based fluorescence trans-glutamination assay, EA inhibited FXIIIa with a moderate potency (IC50 ~ 105 µM) and efficacy (∆Y ~ 66%). In SDS-PAGE experiments, EA appears to significantly inhibit the FXIIIa-mediated polymerization of fibrin(ogen) as well as the formation of fibrin(ogen) - α2-antiplasmin complex which indicates that EA affects the physiological functions of FXIIIa. Interestingly, EA did not affect the clotting times of human plasma in the APTT and the PT assays at the highest concentration tested of 2.5 mM suggesting the lack of effects on the coagulation serine proteases and potentially the functional selectivity of EA with respect to the clotting process. Molecular modeling studies demonstrated that the Michael acceptor of EA forms a covalent bond with catalytic residue of Cys314 in the active site of FXIIIa.
Conclusions: Overall, our studies indicate that EA inhibits the physiological function of human FXIIIa in vitro which may potentially contribute to the bleeding complications that were reported with the association of the parenteral administration of EA.
Keywords: Anticoagulant; Bleeding; Ethacrynic acid; Factor XIIIa; Irreversible inhibitor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII.J Thromb Haemost. 2016 Jul;14(7):1453-61. doi: 10.1111/jth.13354. Epub 2016 Jun 9. J Thromb Haemost. 2016. PMID: 27148673
-
Allosteric Inhibition of Factor XIIIa. Non-Saccharide Glycosaminoglycan Mimetics, but Not Glycosaminoglycans, Exhibit Promising Inhibition Profile.PLoS One. 2016 Jul 28;11(7):e0160189. doi: 10.1371/journal.pone.0160189. eCollection 2016. PLoS One. 2016. PMID: 27467511 Free PMC article.
-
Proteolytic and nonproteolytic activation mechanisms result in conformationally and functionally different forms of coagulation factor XIII A.FEBS J. 2020 Feb;287(3):452-464. doi: 10.1111/febs.15040. Epub 2019 Aug 28. FEBS J. 2020. PMID: 31407850 Free PMC article.
-
Factor XIIIa inhibitors as potential novel drugs for venous thromboembolism.Eur J Med Chem. 2020 Aug 15;200:112442. doi: 10.1016/j.ejmech.2020.112442. Epub 2020 May 18. Eur J Med Chem. 2020. PMID: 32502864 Free PMC article. Review.
-
[Inhibitors of factor XIIIa].Hamostaseologie. 2002 Feb;22(1):43-7. Hamostaseologie. 2002. PMID: 12193984 Review. German.
Cited by
-
Discovery of Heparin Mimetic, Potent, and Selective Inhibitors of Human Clotting Factor XIIIa.ACS Omega. 2024 Jul 3;9(28):31105-31119. doi: 10.1021/acsomega.4c04518. eCollection 2024 Jul 16. ACS Omega. 2024. PMID: 39035933 Free PMC article.
-
Substituted 4H-3,1-benzoxazine-4-one Derivatives as Inhibitors of Cathepsin G.Med Chem. 2024;20(10):944-949. doi: 10.2174/0115734064300678240408084822. Med Chem. 2024. PMID: 38676528 Free PMC article.
References
-
- The US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/16092s042,1609.... Accessed 10 Aug 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

