Depletion of Toxoplasma adenine nucleotide translocator leads to defects in mitochondrial morphology
- PMID: 35642006
- PMCID: PMC9158195
- DOI: 10.1186/s13071-022-05295-7
Depletion of Toxoplasma adenine nucleotide translocator leads to defects in mitochondrial morphology
Abstract
Background: Adenine nucleotide translocase (ANT) is a protein that catalyzes the exchange of ADP/ATP across the inner mitochondrial membrane. Beyond this, ANT is closely associated with cell death pathways and mitochondrial dysfunction. It is a potential therapeutic target for many diseases. The function of the ANT in Toxoplasma gondii is poorly understood.
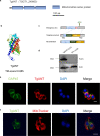
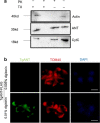
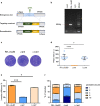
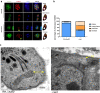
Methods: The CRISPR/CAS9 gene editing tool was used to identify and study the function of the ANT protein in T. gondii. We constructed T. gondii ANT transgenic parasite lines, including endogenous tag strain, knockout strain and gene complement strain, to clarify the function and location of TgANT. Mitochondrial morphology was observed by immunofluorescence and transmission electron microscopy.
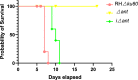
Results: Toxoplasma gondii was found to encode an ANT protein, which was designated TgANT. TgANT localized to the inner mitochondrial membrane. The proliferation of the Δant strain was significantly reduced. More important, depletion of TgANT resulted in significant changes in the morphology and ultrastructure of mitochondria, abnormal apicoplast division and abnormal cytoskeletal daughter budding. In addition, the pathogenicity of the Δant strain to mice was significantly reduced.
Conclusions: Altogether, we identified and characterized the ANT protein of T. gondii. Depletion of TgANT inhibited parasite growth and impaired apicoplast and mitochondrial biogenesis, as well as abnormal parasite division, suggesting TgANT is important for parasite growth.
Keywords: Adenine nucleotide translocase; Mitochondria; Toxoplasma gondii.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Characterization of the apicoplast-localized enzyme TgUroD in Toxoplasma gondii reveals a key role of the apicoplast in heme biosynthesis.J Biol Chem. 2020 Feb 7;295(6):1539-1550. doi: 10.1074/jbc.RA119.011605. Epub 2019 Dec 30. J Biol Chem. 2020. PMID: 31914409 Free PMC article.
-
A new adenine nucleotide transporter located in the ER is essential for maintaining the growth of Toxoplasma gondii.PLoS Pathog. 2022 Jul 5;18(7):e1010665. doi: 10.1371/journal.ppat.1010665. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35788770 Free PMC article.
-
Toxoplasma TgAtg8-TgAtg3 Interaction Primarily Contributes to Apicoplast Inheritance and Parasite Growth in Tachyzoite.Microbiol Spectr. 2022 Feb 23;10(1):e0149521. doi: 10.1128/spectrum.01495-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196797 Free PMC article.
-
Parasite powerhouse: A review of the Toxoplasma gondii mitochondrion.J Eukaryot Microbiol. 2022 Nov;69(6):e12906. doi: 10.1111/jeu.12906. Epub 2022 May 4. J Eukaryot Microbiol. 2022. PMID: 35315174 Free PMC article. Review.
-
The adenine nucleotide translocase 2, a mitochondrial target for anticancer biotherapy.Curr Drug Targets. 2011 Jun;12(6):894-901. doi: 10.2174/138945011795529047. Curr Drug Targets. 2011. PMID: 21269262 Review.
Cited by
-
ATM1, an essential conserved transporter in Apicomplexa, bridges mitochondrial and cytosolic [Fe-S] biogenesis.PLoS Pathog. 2024 Sep 30;20(9):e1012593. doi: 10.1371/journal.ppat.1012593. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39348385 Free PMC article.
-
TgMORN2, a MORN Family Protein Involved in the Regulation of Endoplasmic Reticulum Stress in Toxoplasma gondii.Int J Mol Sci. 2023 Jun 16;24(12):10228. doi: 10.3390/ijms241210228. Int J Mol Sci. 2023. PMID: 37373373 Free PMC article.
-
Metabolic crosstalk between the mitochondrion and the nucleus is essential for Toxoplasma gondii infection.Commun Biol. 2025 Mar 7;8(1):384. doi: 10.1038/s42003-025-07823-4. Commun Biol. 2025. PMID: 40050648 Free PMC article.
-
Iron Stress Affects the Growth and Differentiation of Toxoplasma gondii.Int J Mol Sci. 2024 Feb 21;25(5):2493. doi: 10.3390/ijms25052493. Int J Mol Sci. 2024. PMID: 38473741 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

