Duration of SARS-CoV-2 RNA positivity from various specimens and clinical characteristics in patients with COVID-19: a systematic review and meta-analysis
- PMID: 35642011
- PMCID: PMC9156361
- DOI: 10.1186/s41232-022-00205-x
Duration of SARS-CoV-2 RNA positivity from various specimens and clinical characteristics in patients with COVID-19: a systematic review and meta-analysis
Abstract
Background: The duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA positivity will be important to prevent the spread of coronavirus disease 2019 (COVID-19). A systematic review and meta-analysis were conducted following PRISMA to determine the duration from several parts of the body and clinical characteristics affecting it.
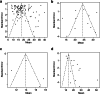
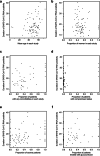
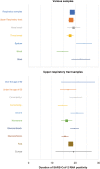
Main text: PubMed, Web of Science, Scopus, and CENTRAL were searched for original studies reporting the duration from COVID-19 onset to the disappearance of viral RNA. Of the 1682 studies identified, 100 met the selection criteria and 13,431 patients were included in this study. The duration of SARS-CoV-2 RNA positivity was 18.29 [95% confidence interval: 17.00-19.89] days in the upper respiratory tract samples, 23.79 [20.43-27.16] days in the sputum, 14.60 [12.16-17.05] days in the blood, and 22.38 [18.40-26.35] days in the stool. Sensitivity analysis revealed that the duration was positively correlated with age, comorbidities, severity, and usage of glucocorticoid. Subgroup analysis indicated that the presence or absence of complications had the greatest impact on the difference in DSRP.
Conclusions: The duration of SARS-CoV-2 RNA positivity was 18.29 days in the upper respiratory tract samples. The duration in the sputum and the stool was longer, while that in the blood was shorter. The duration in the upper respiratory tract samples was longer in older, with any comorbidities, severer, and treated with glucocorticoid. These results provide the basic data for the duration of SARS-CoV-2 RNA positivity, and in the future, the effect of vaccination against SARS-CoV-2 and the SARS-CoV-2 variants on the duration of RNA positivity should be assessed.
Keywords: COVID-19; Coronavirus; Meta-analysis; SARS-CoV-2; SARS-CoV-2 RNA positivity; Systematic review; Viral shedding.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures






Similar articles
-
SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis.Lancet Microbe. 2021 Jan;2(1):e13-e22. doi: 10.1016/S2666-5247(20)30172-5. Epub 2020 Nov 19. Lancet Microbe. 2021. PMID: 33521734 Free PMC article.
-
Duration of viable virus shedding and polymerase chain reaction positivity of the SARS-CoV-2 Omicron variant in the upper respiratory tract: a systematic review and meta-analysis.Int J Infect Dis. 2023 Apr;129:228-235. doi: 10.1016/j.ijid.2023.02.011. Epub 2023 Feb 18. Int J Infect Dis. 2023. PMID: 36804640 Free PMC article.
-
Differences of Severe Acute Respiratory Syndrome Coronavirus 2 Shedding Duration in Sputum and Nasopharyngeal Swab Specimens Among Adult Inpatients With Coronavirus Disease 2019.Chest. 2020 Nov;158(5):1876-1884. doi: 10.1016/j.chest.2020.06.015. Epub 2020 Jun 20. Chest. 2020. PMID: 32569635 Free PMC article.
-
[Shedding of SARS-CoV-2 Virus in COVID-19 Patients and Neutralizing Antibody Level].Mikrobiyol Bul. 2022 Jul;56(3):416-431. doi: 10.5578/mb.20229704. Mikrobiyol Bul. 2022. PMID: 35960235 Turkish.
-
Incidence and Persistence of Viral Shedding in COVID-19 Post-acute Patients With Negativized Pharyngeal Swab: A Systematic Review.Front Med (Lausanne). 2020 Aug 28;7:562. doi: 10.3389/fmed.2020.00562. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32984389 Free PMC article.
Cited by
-
Temporal Dynamics and (Para)Clinical Factors Associated With (Long) Viral RNA Shedding in COVID-19 Nonhospitalized Individuals - The COVID-HOME Study.J Med Virol. 2024 Dec;96(12):e70125. doi: 10.1002/jmv.70125. J Med Virol. 2024. PMID: 39690930 Free PMC article.
-
Time to negative conversion and cardiopulmonary performance in athletes with COVID-19.ERJ Open Res. 2024 Jul 22;10(4):00090-2024. doi: 10.1183/23120541.00090-2024. eCollection 2024 Jul. ERJ Open Res. 2024. PMID: 39040592 Free PMC article.
-
Gut microbiome and clinical and lifestyle host factors associated with recurrent positive RT-PCR for SARS-CoV-2.Front Cell Infect Microbiol. 2024 Dec 18;14:1494193. doi: 10.3389/fcimb.2024.1494193. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39744158 Free PMC article.
-
Viral load dynamics in asymptomatic and symptomatic patients during Omicron BA.2 outbreak in Shanghai, China, 2022: A longitudinal cohort study.Virol Sin. 2024 Dec;39(6):851-859. doi: 10.1016/j.virs.2024.10.001. Epub 2024 Oct 11. Virol Sin. 2024. PMID: 39396663 Free PMC article.
-
SARS-CoV-2 Detection and Culture in Different Biological Specimens from Immunocompetent and Immunosuppressed COVID-19 Patients Infected with Two Different Viral Strains.Viruses. 2023 May 29;15(6):1270. doi: 10.3390/v15061270. Viruses. 2023. PMID: 37376568 Free PMC article.
References
-
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. - DOI - PMC - PubMed
-
- CDC updates and shortens recommended isolation and quarantine period for general population. [cited 2022 February 14th]. Available from: https://www.cdc.gov/media/releases/2021/s1227-isolation-quarantine-guida....
-
- Ministry of Health LaW . Clinical management of patients with COVID-19. A guide for front-line healthcare workers. Version 6.2. 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

