Pentoxifylline alleviates ischemic white matter injury through up-regulating Mertk-mediated myelin clearance
- PMID: 35642056
- PMCID: PMC9153105
- DOI: 10.1186/s12974-022-02480-4
Pentoxifylline alleviates ischemic white matter injury through up-regulating Mertk-mediated myelin clearance
Abstract
Background: Vascular dementia (VAD) is the second most common type of dementia lacking effective treatments. Pentoxifylline (PTX), a nonselective phosphodiesterase inhibitor, displays protective effects in multiple cerebral diseases. In this study, we aimed to investigate the therapeutic effects and potential mechanisms of PTX in VAD.
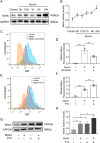
Methods: Bilateral common carotid artery stenosis (BCAS) mouse model was established to mimic VAD. Mouse behavior was tested by open field test, novel object recognition test, Y-maze and Morris water maze (MWM) tests. Histological staining, magnetic resonance imaging (MRI) and electron microscopy were used to define white matter integrity. The impact of PTX on microglia phagocytosis, peroxisome proliferator-activated receptors-γ (PPAR-γ) activation and Mer receptor tyrosine kinase (Mertk) expression was assessed by immunofluorescence, western blotting and flow cytometry with the application of microglia-specific Mertk knockout mice, Mertk inhibitor and PPAR-γ inhibitor.
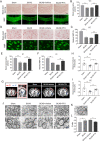
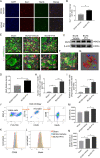
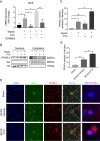
Results: Here, we found that PTX treatment alleviated cognitive impairment in novel object recognition test, Y-maze and Morris water maze tests. Furthermore, PTX alleviated white matter injury in corpus callosum (CC) and internal capsule (IC) areas as shown by histological staining and MRI analysis. PTX-treatment group presented thicker myelin sheath than vehicle group by electron microscopy. Mechanistically, PTX facilitated microglial phagocytosis of myelin debris by up-regulating the expression of Mertk in BCAS model and primary cultured microglia. Importantly, microglia-specific Mertk knockout blocked the therapeutic effects of PTX in BCAS model. Moreover, Mertk expression was regulated by the nuclear translocation of PPAR-γ. Through modulating PPAR-γ, PTX enhanced Mertk expression.
Conclusions: Collectively, our results demonstrated that PTX showed therapeutic potentials in VAD and alleviated ischemic white matter injury via modulating Mertk-mediated myelin clearance in microglia.
Keywords: Mertk; Microglia; PPAR-γ; Pentoxifylline; Phagocytosis; Vascular dementia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134:226–239. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

