The separation of several organophosphate pesticides on immobilized polysaccharide chiral stationary phases
- PMID: 35642080
- PMCID: PMC9544164
- DOI: 10.1002/chir.23473
The separation of several organophosphate pesticides on immobilized polysaccharide chiral stationary phases
Abstract
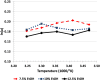
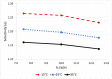
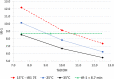
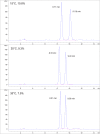
While not initially a focus or priority, in recent decades, an emphasis has been placed on the activity of individual enantiomers of widely used pesticides. Of particular note are organophosphorus-based pesticides like fenamiphos and profenofos, as examples. This work explores the enantioselective high-performance liquid chromatography (HPLC) separations of seven such organophosphorus pesticides (OP's) on the library of immobilized polysaccharide-based chiral stationary phases (CSPs) with normal phase hexane/alcohol mixtures. Further exploration of the effect of mobile phase strength and temperature on several of the separations was performed using simple factorial design. Equivalent retention of the first eluting enantiomer of several combinations of temperature and mobile phase was compared for peak shape, selectivity, and resolution. Similarly, equivalent selectivity of several combinations of temperature and mobile phase was compared for peak shape, retention of the first eluting enantiomer, and resolution. The results of this study make available several new chiral separations of the OPs included in the work that were not previously documented, including separations on the three most recently commercialized phases, Chiralpak IH, IJ, and IK. Additionally, sufficient understanding was obtained to be able to predict the trade-off of resolution, analysis time, peak sharpness (and thus improve limit-of-detection [LOD]/limit-of-quantification [LOQ]), robustness, and convenience of conditions for further application optimization.
Keywords: chiral HPLC; normal phase HPLC; organo-phosphate pesticides; polysaccharides.
© 2022 The Authors. Chirality published by Wiley Periodicals LLC.
Figures






Similar articles
-
High-performance liquid chromatographic separation of the enantiomers of organophosphorus pesticides on polysaccharide chiral stationary phases.J Chromatogr A. 2001 Sep 14;928(2):145-54. doi: 10.1016/s0021-9673(01)01138-4. J Chromatogr A. 2001. PMID: 11587332
-
High-performance liquid chromatographic enantioseparation of isopulegol-based ß-amino lactone and ß-amino amide analogs on polysaccharide-based chiral stationary phases focusing on the change of the enantiomer elution order.J Chromatogr A. 2020 Jun 21;1621:461054. doi: 10.1016/j.chroma.2020.461054. Epub 2020 Mar 17. J Chromatogr A. 2020. PMID: 32204880
-
[Comparative enantiomer separation of beta-blockers on polysaccharide derived chiral stationary phases using high performance liquid chromatography with acid or base additive in the mobile phases].Se Pu. 2009 Jul;27(4):467-71. Se Pu. 2009. PMID: 19938505 Chinese.
-
Polysaccharide-Based Chiral Stationary Phases for Enantioseparations by High-Performance Liquid Chromatography: An Overview.Methods Mol Biol. 2019;1985:93-126. doi: 10.1007/978-1-4939-9438-0_6. Methods Mol Biol. 2019. PMID: 31069731 Review.
-
Synthesis and application of immobilized polysaccharide-based chiral stationary phases for enantioseparation by high-performance liquid chromatography.J Chromatogr A. 2014 Oct 10;1363:51-61. doi: 10.1016/j.chroma.2014.06.042. Epub 2014 Jun 22. J Chromatogr A. 2014. PMID: 24997110 Review.
Cited by
-
Development and application of chiral separation technology based on chiral metal-organic frameworks.J Pharm Anal. 2025 Jul;15(7):101176. doi: 10.1016/j.jpha.2024.101176. Epub 2024 Dec 28. J Pharm Anal. 2025. PMID: 40735030 Free PMC article. Review.
References
-
- Unsworth J. History of pesticide use. IUPAC. 2010. https://agrochemicals.iupac.org/index.php?option=com_sobi2&sobi2Task=sob...
-
- Erbach G. Pesticide legislation in the EU. European Parliament. 2012;1‐6. https://www.europarl.europa.eu/RegData/bibliotheque/briefing/2012/120291...
-
- Li L, Zhou S, Jin L, Zhang C, Liu W. Enantiomeric separation of organophosphorus pesticides by high‐performance liquid chromatography, gas chromatography and capillary electrophoresis and their applications to environmental fate and toxicity assays. J Chromatogr B. 2010;878(17):1264‐1276. doi:10.1016/j.jchromb.2009.10.031 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

