Current Perspectives in ABO-Incompatible Kidney Transplant
- PMID: 35642217
- PMCID: PMC9148605
- DOI: 10.2147/JIR.S360460
Current Perspectives in ABO-Incompatible Kidney Transplant
Abstract
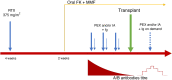
For a long time, ABO incompatible living donor kidney transplantation has been considered contraindicated, due to the presence of isohemagglutinins, natural antibodies reacting with non-self ABO antigens. However, as the demand for kidney transplantation is constantly growing, methods to expand the donor pool have become increasingly important. Thus, in the last decades, specific desensitization strategies for ABOi transplantation have been developed. Nowadays, these regimens consist of transient removal of preformed anti-A or anti-B antibodies by using plasmapheresis or immunoadsorption and B-cell immunity modulation by CD20+ cells depletion with rituximab, in association with maintenance immunosuppression including corticosteroids, tacrolimus and mycophenolate mofetil. The outcome in ABOi kidney transplantation have markedly improved over the years. In fact, although randomized trials are still lacking, recent meta analysis has revealed that there is no difference in terms of graft and patient's survival between ABOi and ABO compatible kidney transplant, even in the long term. However, many concerns still exist, because ABOi kidney transplantation is associated with an increased risk of bleeding and infectious complications, partly related to the effects of extracorporeal treatments and the strong immunosuppression. Thus, a continuous improvement in desensitization strategies, with the aim of minimize the immunosuppressive burden, on the basis of immune pathogenesis, antibodies titers and/or ABO blood group, is warranted. In this review, we discuss the main immune mechanisms involved in ABOi kidney transplantation, the pathogenesis of tolerance and the desensitization regimens, including immunoadsorption and plasmapheresis and the immunosuppressive protocol. Finally, we provide an overview on outcome and future perspectives in ABOi kidney transplant.
Keywords: ABO incompatible kidney transplant; blood group; plasma exchange; rituximab.
© 2022 Maritati et al.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Alexander JW, Zola JC. Expanding the donor pool: use of marginal donors for solid organ transplantation. Clin Transplant. 1996;10(1 Pt 1):1–19. - PubMed
-
- Ministry of Health. Transplants. Italian and European data.Available from: http://www.trapianti.salute.gov.it/trapianti/archivioDatiCnt.jsp. Accessed May 11, 2022.
Publication types
LinkOut - more resources
Full Text Sources

