Neuroretinitis after the second injection of a SARS-CoV-2-vaccine: A case report
- PMID: 35642221
- PMCID: PMC9132495
- DOI: 10.1016/j.ajoc.2022.101592
Neuroretinitis after the second injection of a SARS-CoV-2-vaccine: A case report
Abstract
Purpose: We report the first case of neuroretinitis after administration of a second dose of a messenger RNA vaccine for coronavirus disease-2019 (COVID-19).
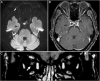
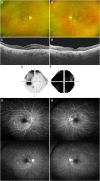
Observations: An 83-year-old healthy woman presented with subacute, painless, and progressive visual loss in the right eye that started 2 days after the second injection of the COVID-19 vaccine (Comirnaty®) from Pfizer (New York, NY, USA) and BioNTech (Mainz, Germany). Visual acuities were hand motion perception in the right eye and 20/30 in the left eye. There was optic nerve head swelling in the right eye and subretinal fluid and disruption of the photoreceptor layers in both eyes. Magnetic resonance imaging revealed an enhancement of the right optic nerve, consistent with optic neuritis. She was treated with intravenous corticosteroids, and the optic nerve swelling in the right eye resolved promptly. However, the amount of subretinal fluid worsened for 1 month and did not improve until 6 months from onset. Her visual acuity was slightly improved to finger count perception in the right eye and 20/20 in the left eye during an examination 6 months from onset.
Conclusions and importance: Considering the temporal relation between the second dose of vaccination and the symptom onset in our patient, the ophthalmic symptoms here reported might be considered a rare adverse effect of the Comirnaty® COVID-19 vaccine. Although a causal relationship is not established, to our knowledge, this is the first report of neuroretinitis after vaccination with Comirnaty®, and any further similar cases should be examined in detail.
Keywords: Neuroretinitis; SARS-CoV-2-vaccine.
© 2022 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- FDA. Fact sheet for healthcare providers administering vaccine (vaccination providers): Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Revised January 2021. Available at http://lps3.www.fda.gov.libproxy.samsunghospital.com/media/144413/download. Accessed January 17, 2021.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

