Continuous Infusion of Factor VIII and von Willebrand Factor in Surgery: Trials with pdFVIII LFB or pdVWF LFB in Patients with Bleeding Disorders
- PMID: 35642281
- PMCID: PMC9393085
- DOI: 10.1055/a-1865-6978
Continuous Infusion of Factor VIII and von Willebrand Factor in Surgery: Trials with pdFVIII LFB or pdVWF LFB in Patients with Bleeding Disorders
Abstract
Background: A plasma-derived factor VIII product (pdFVIII; Factane 100 or 200 IU/mL) and a plasma-derived von Willebrand factor product (pdVWF; Wilfactin 100 IU/mL) are approved for replacement therapy by intravenous bolus injections in hemophilia A (HA) and von Willebrand disease (VWD), respectively. However, in situations requiring intensive treatment, continuous infusion (CI) may be desirable to better control target plasma factor levels.
Aim: To evaluate the perioperative hemostatic efficacy and safety of these concentrates administered by CI.
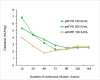
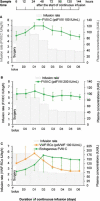
Methods: Three phase III trials were conducted. Adults with HA (FVIII:C < 1%) (studies 1 and 2) or VWD (VWF:RCo < 20%) (Study 3) received a preoperative bolus followed by CI of undiluted concentrate for at least 6 days. Bolus doses and CI rates were based on individual recovery and clearance, respectively. The initial infusion rate had to be higher for 48 hours for HA and 24 hours for VWD patients to anticipate potential fluctuations of factor concentrations during major surgery. Target levels of FVIII:C in HA and VWF:RCo in VWD were 80 and 70 IU/dL, respectively. Efficacy was assessed using a global hemostatic efficacy score.
Results: Studies 1, 2, and 3 included 12, 4, and 6 patients, respectively. Efficacy outcomes were excellent/good in all 22 major surgeries including 18 orthopedic procedures. Most daily measured FVIII and VWF levels (92%) were on target. No safety concerns, thrombotic events, or inhibitors were identified.
Conclusion: pdFVIII and pdVWF administered by CI represent an effective and safe alternative to bolus injections in patients with severe HA or VWD undergoing surgery.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
J.W. received grant support from Alnylam Pharmaceuticals, Baxalta, LFB, Novo Nordisk, Octapharma, Rigel Pharmaceuticals, Roche, Shire/Takeda, and Sobi; also sponsored lectures from Alexion, Baxalta, CSL Behring, Ferring Pharmaceuticals, Novo Nordisk, Octapharma, Roche, Sanofi/Genzyme, Shire/Takeda, Siemens, Sobi, and Werfen. B.G. has received grant support from CSL Behring, Octapharma and Sobi; also sponsored lectures from CSL Behring, Novo Nordisk, Roche, and Shire/Takeda. L.R. has no conflicts of interest to declare. E.S.-W. has no conflicts of interest to declare. V.C. has received honoraria or consultation fees from Roche, Novo Nordisk, and Sobi, also support for attending scientific meetings from LFB and Sobi. S.P. is an employee of LFB; C.H. is an employee of LFB. F.B. is a former employee of LFB. C.N. received research grant or honoraria or participated in clinical trials from Alnylam/Sanofi, Bayer, Biomarin, Bioverativ/Sobi, Bayer, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche/Spark, and Shire/Takeda.
Figures
References
-
- Luck J V, Jr, Kasper C K. Surgical management of advanced hemophilic arthropathy. An overview of 20 years' experience. Clin Orthop Relat Res. 1989;(242):60–82. - PubMed
-
- Leebeek F WG, Eikenboom J CJ. Von Willebrand's disease. N Engl J Med. 2016;375(21):2067–2080. - PubMed
-
- WiN Study Group . van Galen K P, Timmer M, de Kleijn P. Long-term outcome after joint bleeds in von Willebrand disease compared to haemophilia A: a post hoc analysis. Thromb Haemost. 2018;118(10):1690–1700. - PubMed
-
- European Wilate® Study Group . Windyga J, von Depka-Prondzinski M. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (Wilate®) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost. 2011;105(06):1072–1079. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

