ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors
- PMID: 35642431
- PMCID: PMC9381089
- DOI: 10.1158/1535-7163.MCT-21-0851
ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors
Abstract
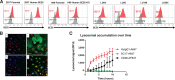
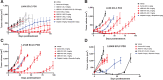
In the past year, four antibody-drug conjugates (ADC) were approved, nearly doubling the marketed ADCs in oncology. Among other attributes, successful ADCs optimize targeting antibody, conjugation chemistry, and payload mechanism of action. Here, we describe the development of ABBV-011, a novel SEZ6-targeted, calicheamicin-based ADC for the treatment of small cell lung cancer (SCLC). We engineered a calicheamicin conjugate that lacks the acid-labile hydrazine linker that leads to systemic release of a toxic catabolite. We then screened a patient-derived xenograft library to identify SCLC as a tumor type with enhanced sensitivity to calicheamicin ADCs. Using RNA sequencing (RNA-seq) data from primary and xenograft SCLC samples, we identified seizure-related homolog 6 (SEZ6) as a surface-expressed SCLC target with broad expression in SCLC and minimal normal tissue expression by both RNA-seq and IHC. We developed an antibody targeting SEZ6 that is rapidly internalized upon receptor binding and, when conjugated to the calicheamicin linker drug, drives potent tumor regression in vitro and in vivo. These preclinical data suggest that ABBV-011 may provide a novel treatment for patients with SCLC and a rationale for ongoing phase I studies (NCT03639194).
©2022 The Authors; Published by the American Association for Cancer Research.
Figures




Comment in
- Mol Cancer Ther. 21:857.
- Mol Cancer Ther. 21:857.
References
-
- Lee M, Ellestad GA, Borders DB. Calicheamicins: discovery, structure, chemistry, and interaction with DNA. Acc Chem Res 1991;24.8:235–43.
-
- Nicolaou KC, Rigol S. The role of organic synthesis in the emergence and development of antibody-drug conjugates as targeted cancer therapies. Angew Chem Int Ed Engl 2019;58:11206–41. - PubMed
-
- Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood 2017;130:2373–6. - PubMed
-
- Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, et al. . Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem 2002;13:47–58. - PubMed
-
- Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 2001;41:1206–14. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

