Performance of nasopharyngeal swab and saliva in detecting Delta and Omicron SARS-CoV-2 variants
- PMID: 35642439
- PMCID: PMC9348014
- DOI: 10.1002/jmv.27898
Performance of nasopharyngeal swab and saliva in detecting Delta and Omicron SARS-CoV-2 variants
Abstract
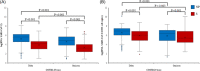
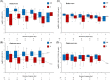
A prospective cohort study was conducted during the Delta and Omicron severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) epidemic waves from paired nasopharyngeal swab (NPS or NP swab) and saliva samples taken from 624 participants. The study aimed to assess if any differences among participants from both waves could be observed and if any difference in molecular diagnostic performance could be observed among the two sample types. Samples were transported immediately to the laboratory to ensure the highest possible sample quality without any freezing and thawing steps before processing. Nucleic acids from saliva and NPS were prospectively extracted and SARS-CoV-2 was detected using a real-time reverse-transcription polymerase chain reaction. All observed results were statistically analyzed. Although the results obtained with NP and saliva agreed overall, higher viral loads were observed in NP swabs regardless of the day of specimen collection in both SARS-CoV-2 epidemic waves. No significant difference could be observed between the two epidemic waves characterized by Delta or Omicron SARS-CoV-2. To note, Delta infection resulted in higher viral loads both in NP and saliva and more symptoms, including rhinorrhea, cough, and dyspnea, whereas Omicron wave patients more frequently reported sore throat. An increase in the mean log RNA of SARS-CoV-2 was observed with the number of expressed symptoms in both waves, however, the difference was not significant. Data confirmed that results from saliva were concordant with those from NP swabs, although saliva proved to be a challenging sample with frequent inhibitions that required substantial retesting.
Keywords: COVID-19; SARS-CoV-2 diagnostics; nasopharyngeal swab; real-time RT-PCR; saliva.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

