Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies
- PMID: 35642485
- PMCID: PMC9152964
- DOI: 10.3324/haematol.2021.279512
Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies
Abstract
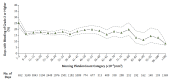
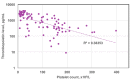
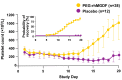
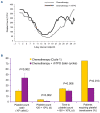
Chemotherapy-induced thrombocytopenia (CIT) is a common complication of the treatment of non-hematologic malignancies. Many patient-related variables (e.g., age, tumor type, number of prior chemotherapy cycles, amount of bone marrow tumor involvement) determine the extent of CIT. CIT is related to the type and dose of chemotherapy, with regimens containing gemcitabine, platinum, or temozolomide producing it most commonly. Bleeding and the need for platelet transfusions in CIT are rather uncommon except in patients with platelet counts below 25x109/L in whom bleeding rates increase significantly and platelet transfusions are the only treatment. Nonetheless, platelet counts below 70x109/L present a challenge. In patients with such counts, it is important to exclude other causes of thrombocytopenia (medications, infection, thrombotic microangiopathy, post-transfusion purpura, coagulopathy and immune thrombocytopenia). If these are not present, the common approach is to reduce chemotherapy dose intensity or switch to other agents. Unfortunately decreasing relative dose intensity is associated with reduced tumor response and remission rates. Thrombopoietic growth factors (recombinant human thrombopoietin, pegylated human megakaryocyte growth and development factor, romiplostim, eltrombopag, avatrombopag and hetrombopag) improve pretreatment and nadir platelet counts, reduce the need for platelet transfusions, and enable chemotherapy dose intensity to be maintained. National Comprehensive Cancer Network guidelines permit their use but their widespread adoption awaits adequate phase III randomized, placebo-controlled studies demonstrating maintenance of relative dose intensity, reduction of platelet transfusions and bleeding, and possibly improved survival. Their potential appropriate use also depends on consensus by the oncology community as to what constitutes an appropriate pretreatment platelet count as well as identification of patient-related and treatment variables that might predict bleeding.
Figures


References
-
- Kuter D. General aspects of thrombocytopenia, platelet transfusions, and thrombopoietic growth factors. In: Kitchens CS, Kessler CM, Konkle BA, et al.. editors. Consultative Hemostasis and Thrombosis, 4th edition. Philadelphia: Elsevier; 2019:108-126.
-
- Kuter D. Clinical applications of thrombopoietic growth factors. In: Plow T, editor. UpToDate. http://www.uptodate.com (accessed October 26, 2021).
-
- Kuter DJ. What is the role of novel thrombopoietic agents in the management of acute leukemia? Best Pract Res Clin Haematol. 2016;29(4):372-378. - PubMed
-
- National Institutes of Health NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In: U.S: Department of Health and Human Services, 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

