Secnidazole: a treatment for trichomoniasis in adolescents and adults
- PMID: 35642509
- PMCID: PMC9844242
- DOI: 10.1080/14787210.2022.2080656
Secnidazole: a treatment for trichomoniasis in adolescents and adults
Abstract
Introduction: Single-dose 2-g oral secnidazole (SEC), newly approved by the U.S. Food and Drug administration (FDA) for treatment of trichomoniasis, is a potent 5-nitroimidazole with selective toxicity against various micro-organisms. It has been used internationally to treat trichomoniasis, bacterial vaginosis, and other infections for decades. Trichomoniasis is the most common non-viral sexually transmitted infection worldwide and is associated with significant morbidity. In comparison to the only other FDA-approved treatments for trichomoniasis in the United States - metronidazole and tinidazole - SEC has favorable pharmacokinetics, including a longer half-life and a lower minimal lethal concentration.
Areas covered: This work summarizes the chemistry and pharmacology of SEC and reviews the evidence on its efficacy, tolerability, and safety for the treatment of trichomoniasis.
Expert opinion: SEC is an efficacious, well tolerated, and safe treatment for patients aged ≥12 years with trichomoniasis. Single-dose administration makes it a favorable treatment option, especially in cases where adherence to multi-dose treatment regimens may be low.
Keywords: 5-nitroimidazoles; Trichomonas vaginalis; Trichomoniasis; metronidazole; secnidazole; sexually transmitted infections; tinidazole.
Conflict of interest statement
Declaration of interest
C Muzny has received research grant support from Lupin Pharmaceuticals, Gilead Sciences, Inc, and Abbott Molecular, is a consultant for Lupin Pharmaceuticals, PhagoMed, and BioFire Diagnostics, and has received honoraria from Elsevier, Abbott Molecular, Cepheid, Becton Dickinson, Roche Diagnostics, and Lupin. O Van Gerwen has received research grant support from Gilead Sciences, Inc and Abbott Molecular.
Figures
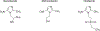

Similar articles
-
Secnidazole for Trichomoniasis in Women and Men.Sex Med Rev. 2022 Apr;10(2):255-262. doi: 10.1016/j.sxmr.2021.12.004. Epub 2022 Feb 10. Sex Med Rev. 2022. PMID: 35153156 Free PMC article. Review.
-
Nitroimidazoles in the treatment of trichomoniasis, giardiasis, and amebiasis.Int J Clin Pharmacol Ther Toxicol. 1984 Feb;22(2):63-72. Int J Clin Pharmacol Ther Toxicol. 1984. PMID: 6698665
-
Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis.Drugs. 1996 Apr;51(4):621-38. doi: 10.2165/00003495-199651040-00007. Drugs. 1996. PMID: 8706597 Review.
-
Successful treatment with secnidazole for trichomoniasis in the setting of metronidazole hypersensitivity.Int J STD AIDS. 2021 Nov;32(13):1271-1273. doi: 10.1177/09564624211034974. Epub 2021 Aug 15. Int J STD AIDS. 2021. PMID: 34392726
-
Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis.J Obstet Gynaecol Can. 2015 Mar;37(3):266-274. doi: 10.1016/S1701-2163(15)30316-9. J Obstet Gynaecol Can. 2015. PMID: 26001874
Cited by
-
Trichomonas vaginalis Virus: Current Insights and Emerging Perspectives.Viruses. 2025 Jun 26;17(7):898. doi: 10.3390/v17070898. Viruses. 2025. PMID: 40733518 Free PMC article. Review.
-
Trichomoniasis.Infect Dis Clin North Am. 2023 Jun;37(2):245-265. doi: 10.1016/j.idc.2023.02.001. Epub 2023 Mar 31. Infect Dis Clin North Am. 2023. PMID: 37005163 Free PMC article. Review.
-
Investigation of the frequency and relationship between trichomonas infection in the preterm delivery (a case-control study in Amir Al-Momenin Hospital, Semnan).J Family Med Prim Care. 2024 Apr;13(4):1362-1370. doi: 10.4103/jfmpc.jfmpc_1411_23. Epub 2024 Apr 22. J Family Med Prim Care. 2024. PMID: 38827714 Free PMC article.
-
Trichomoniasis and Other Sexually Transmitted Parasitic Diseases in Women.Clin Obstet Gynecol. 2025 Jun 1;68(2):194-205. doi: 10.1097/GRF.0000000000000945. Epub 2025 Apr 14. Clin Obstet Gynecol. 2025. PMID: 40226933 Free PMC article. Review.
-
Efficacy of single-dose oral secnidazole for the treatment of trichomoniasis in women co-infected with trichomoniasis and bacterial vaginosis: a post hoc subgroup analysis of phase 3 clinical trial data.BMJ Open. 2023 Aug 7;13(8):e072071. doi: 10.1136/bmjopen-2023-072071. BMJ Open. 2023. PMID: 37550019 Free PMC article. Clinical Trial.
References
-
-
Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187.
**Recently updated 2021 CDC STI Treatment Guidelines.
-
-
-
Committee on Practice Bulletins-Gynecology. Vaginitis in Nonpregnant Patients: ACOG Practice Bulletin, Number 215. Obstet Gynecol. 2020;135(1):e1–e17.
**Recently updated ACOG Guidelines for the Treatment of Vaginitis in Nonpregnant Patients.
-
-
- Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):519–22. - PubMed
-
- Yagur Y, Weitzner O, Tiosano LB, et al. Characteristics of Pelvic Inflammatory Disease caused by Sexually transmitted disease - An epidemiologic Study. J Gynecol Obstet Hum Reprod. 2021:102176. - PubMed
-
- Yang S, Zhao W, Wang H, et al. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2018;228:166–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
