Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021
- PMID: 35642775
- PMCID: PMC9253749
- DOI: 10.1111/cns.13867
Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021
Abstract
Background: To assess the temporal changes in the characteristics of ischemic stroke drug clinical trials conducted in mainland China in 2005-2021.
Methods: A statistical analysis of registered clinical trials on ischemic stroke was performed using the platform of the Center for Drug Evaluation of China National Medical Products Administration, the Chinese Clinical Trial Registry, and ClinicalTrials.gov websites.
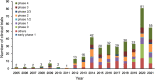
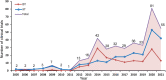
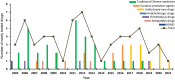
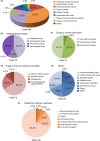
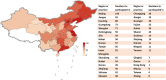
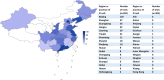
Results: From January 1, 2005 to August 1, 2021, a total of 384 registered drug clinical trials on ischemic stroke were identified in mainland China. Over time, the number of trials gradually increased each year, with a significant growth in 2014, from 16 in 2013 to 42 in 2014. Phase IV trials (31.8%) accounted for the majority, followed by phase II (16.4%), phase I (10.9%), and phase III (8.6%). In terms of sponsorship, the proportion of investigator-initiated trials (IITs) (60.7%) was higher than industry-sponsored trials (ISTs) (39.3%). Additionally, trials involving traditional Chinese medicines (TCMs) (36.2%) accounted for the largest proportion, followed by trials involving antithrombotic therapy (19.5%) and cerebral protection agents (16.7%). Furthermore, over the past 17 years, the number of leading drug clinical trial units for ischemic stroke in mainland China has continuously increased. The leading principal units from Beijing, Shanghai, Guangdong, Jiangsu, and Liaoning accounted for the majority of the trials (67.4%).
Conclusion: In the past 17 years, great progress has been made in the research and development (R&D) of drugs and clinical trials for ischemic stroke in mainland China. The most extensive progress was observed in TCMs, antithrombotic therapy, and cerebral protection agents. More clinical trials are needed to confirm whether the newly developed drugs can improve the clinical efficacy of ischemic stroke. Simultaneously, more pharmaceutical R&D efforts of innovative drugs are warranted.
Keywords: drug clinical trials; ischemic stroke; mainland China.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a Nationwide population‐based survey of 480 687 adults. Circulation. 2017;135(8):759‐771. - PubMed
-
- Wang D, Liu J, Liu M, Lu C, Brainin M, Zhang J. Patterns of stroke between university hospitals and nonuniversity hospitals in mainland China: prospective multicenter hospital‐based registry study. World Neurosurg. 2017;98:258‐265. - PubMed
-
- Huang Y, Wang JG, Wei JW, et al. Age and gender variations in the management of ischaemic stroke in China. Int J Stroke. 2010;5(5):351‐359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

