Cardiovascular deconditioning increases GABA signaling in the nucleus tractus solitarii
- PMID: 35642806
- PMCID: PMC9236861
- DOI: 10.1152/jn.00102.2022
Cardiovascular deconditioning increases GABA signaling in the nucleus tractus solitarii
Abstract
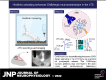
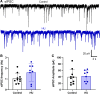
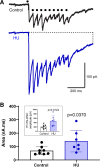
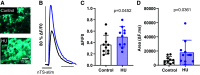
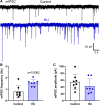
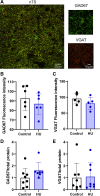
The nucleus tractus solitarii (nTS) is the major integrative brainstem region for autonomic modulation and processing of cardiovascular reflexes. GABA and glutamate are the main inhibitory and excitatory neurotransmitters, respectively, within this nucleus. Alterations in the GABA-glutamate regulation in the nTS are related to numerous cardiovascular comorbidities. Bedridden individuals and people exposed to microgravity exhibit dysautonomia and cardiovascular deconditioning that are mimicked in the hindlimb unloading (HU) rat model. We have previously shown in the nTS that HU increases glutamatergic neurotransmission yet decreases neuronal excitability. In this study, we investigated the effects of HU on nTS GABAergic neurotransmission. We hypothesized that HU potentiates GABA signaling via increased GABAergic release and postsynaptic GABA receptor expression. Following HU or control postural exposure, GABAergic neurotransmission was assessed using whole cell patch clamp whereas the magnitude of GABA release was evaluated via an intensity-based GABA sensing fluorescence reporter (iGABASnFR). In response to GABA interneuron stimulation, the evoked inhibitory postsynaptic current (nTS-IPSC) amplitude and area, as well as iGABASnFR fluorescence, were greater in HU than in control. HU also elevated the frequency but not the amplitude of spontaneous miniature IPSCs. Picoapplication of GABA produced similar postsynaptic current responses in nTS neurons of HU and control. Moreover, HU did not alter GABAA receptor α1 subunit expression, indicating minimal alterations in postsynaptic membrane receptor expression. These results indicate that HU increases GABAergic signaling in the nTS likely via augmented release of GABA from presynaptic terminals. Altogether, our data indicate GABA plasticity contributes to the autonomic and cardiovascular alterations following cardiovascular deconditioning (CVD).NEW & NOTEWORTHY Gravity influences distribution of blood volume and autonomic function. Microgravity and prolonged bed rest induce cardiovascular deconditioning (CVD). We used hindlimb unloading (HU), a rat analog for bed rest, to investigate CVD-induced neuroplasticity in the brainstem. Our data demonstrate that HU increases GABA modulation of nucleus tractus solitarii (nTS) neurons via presynaptic plasticity. Given the importance of nTS in integrating cardiovascular reflexes, this study provides new evidence on the central mechanisms behind CVD following HU.
Keywords: IPSC; autonomic nervous system; hindlimb unloading; synaptic transmission.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

