Nanomaterial Inhalation During Pregnancy Alters Systemic Vascular Function in a Cyclooxygenase-Dependent Manner
- PMID: 35642938
- PMCID: PMC9333412
- DOI: 10.1093/toxsci/kfac055
Nanomaterial Inhalation During Pregnancy Alters Systemic Vascular Function in a Cyclooxygenase-Dependent Manner
Abstract
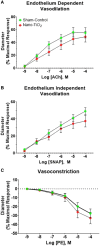
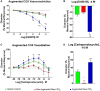
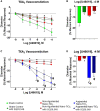
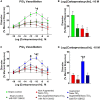
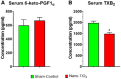
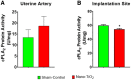
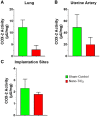
Pregnancy requires rapid adaptations in the uterine microcirculation to support fetal development. Nanomaterial inhalation is associated with cardiovascular dysfunction, which may impair gestation. We have shown that maternal nano-titanium dioxide (nano-TiO2) inhalation impairs microvascular endothelial function in response to arachidonic acid and thromboxane (TXA2) mimetics. However, the mechanisms underpinning this process are unknown. Therefore, we hypothesize that maternal nano-TiO2 inhalation during gestation results in uterine microvascular prostacyclin (PGI2) and TXA2 dysfunction. Pregnant Sprague-Dawley rats were exposed from gestational day 10-19 to nano-TiO2 aerosols (12.17 ± 1.67 mg/m3) or filtered air (sham-control). Dams were euthanized on gestational day 20, and serum, uterine radial arterioles, implantation sites, and lungs were collected. Serum was assessed for PGI2 and TXA2 metabolites. TXB2, the stable TXA2 metabolite, was significantly decreased in nano-TiO2 exposed dams (597.3 ± 84.4 vs 667.6 ± 45.6 pg/ml), whereas no difference was observed for 6-keto-PGF1α, the stable PGI2 metabolite. Radial arteriole pressure myography revealed that nano-TiO2 exposure caused increased vasoconstriction to the TXA2 mimetic, U46619, compared with sham-controls (-41.3% ± 4.3% vs -16.8% ± 3.4%). Nano-TiO2 exposure diminished endothelium-dependent vasodilation to carbaprostacyclin, a PGI2 receptor agonist, compared with sham-controls (30.0% ± 9.0% vs 53.7% ± 6.0%). Maternal nano-TiO2 inhalation during gestation decreased nano-TiO2 female pup weight when compared with sham-control males (3.633 ± 0.064 vs 3.995 ± 0.124 g). Augmented TXA2 vasoconstriction and decreased PGI2 vasodilation may lead to decreased placental blood flow and compromise maternofetal exchange of waste and nutrients, which could ultimately impact fetal health outcomes.
Keywords: advanced materials; microcirculation; prostacyclin; thromboxane; titanium dioxide; uterine arterioles.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

