The European Medicines Agency review of sacituzumab govitecan for the treatment of triple-negative breast cancer
- PMID: 35642987
- PMCID: PMC9149193
- DOI: 10.1016/j.esmoop.2022.100497
The European Medicines Agency review of sacituzumab govitecan for the treatment of triple-negative breast cancer
Abstract
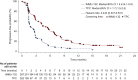
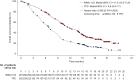
Sacituzumab govitecan (SG) is an antineoplastic agent which combines a humanized monoclonal antibody binding to trophoblast cell surface antigen-2 (Trop-2)-expressing cancer cells, linked with cytotoxic moiety SN-38 (govitecan) with topoisomerase I inhibitor action. On 22 November 2021, a marketing authorization valid through the European Union (EU) was issued under the European Medicines Agency (EMA)'s accelerated assessment program for SG as monotherapy for the treatment of adult patients with unresectable or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, including at least one of them for advanced disease. The assessment was based on results from an open-label, randomized, phase III trial to evaluate the safety, tolerability, pharmacokinetics and efficacy of SG versus treatment of physician's choice (TPC) in patients with mTNBC who received at least two prior treatments including at least one of them for advanced disease. The efficacy results in the overall population, based on mature data, showed a statistically significant improvement of SG over TPC in progression-free survival (PFS) and overall survival (OS). The median PFS was 4.8 months versus 1.7 months [hazard ratio (HR) = 0.43, n = 529; 95% CI 0.35-0.54; P < 0.0001] and the median OS was 11.8 months versus 6.9 months (HR = 0.51, n = 529; 95% CI 0.41-0.62; P < 0.0001). The most common (>30%) side effects of SG were diarrhea, neutropenia, nausea, fatigue, alopecia, anemia, constipation and vomiting. The aim of this manuscript is to summarize the scientific review of the application leading to regulatory approval in the EU.
Keywords: ADC; EMA; TNBC; Trop-2; sacituzumab govitecan.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures
Similar articles
-
Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer.N Engl J Med. 2021 Apr 22;384(16):1529-1541. doi: 10.1056/NEJMoa2028485. N Engl J Med. 2021. PMID: 33882206 Clinical Trial.
-
Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer.N Engl J Med. 2019 Feb 21;380(8):741-751. doi: 10.1056/NEJMoa1814213. N Engl J Med. 2019. PMID: 30786188 Clinical Trial.
-
Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer.Ann Oncol. 2021 Sep;32(9):1148-1156. doi: 10.1016/j.annonc.2021.06.002. Epub 2021 Jun 8. Ann Oncol. 2021. PMID: 34116144 Clinical Trial.
-
Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer.Expert Rev Anticancer Ther. 2019 Aug;19(8):673-679. doi: 10.1080/14737140.2019.1654378. Epub 2019 Aug 19. Expert Rev Anticancer Ther. 2019. PMID: 31398063 Review.
-
Sacituzumab Govitecan-hziy: An Antibody-Drug Conjugate for the Treatment of Refractory, Metastatic, Triple-Negative Breast Cancer.Ann Pharmacother. 2021 Jul;55(7):921-931. doi: 10.1177/1060028020966548. Epub 2020 Oct 17. Ann Pharmacother. 2021. PMID: 33070624 Review.
Cited by
-
Preliminary results from ASCENT-J02: a phase 1/2 study of sacituzumab govitecan in Japanese patients with advanced solid tumors.Int J Clin Oncol. 2024 Nov;29(11):1684-1695. doi: 10.1007/s10147-024-02589-x. Epub 2024 Sep 20. Int J Clin Oncol. 2024. PMID: 39302614 Free PMC article. Clinical Trial.
-
m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer.J Mol Endocrinol. 2022 Dec 21;70(2):e220110. doi: 10.1530/JME-22-0110. Print 2023 Feb 1. J Mol Endocrinol. 2022. PMID: 36367225 Free PMC article. Review.
-
Biomarkers in breast cancer 2024: an updated consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology.Clin Transl Oncol. 2024 Dec;26(12):2935-2951. doi: 10.1007/s12094-024-03541-1. Epub 2024 Jun 13. Clin Transl Oncol. 2024. PMID: 38869741 Free PMC article.
-
TRPML1 as a potential therapeutic target for triple-negative breast cancer: a review.Front Oncol. 2023 Dec 13;13:1326023. doi: 10.3389/fonc.2023.1326023. eCollection 2023. Front Oncol. 2023. PMID: 38156109 Free PMC article. Review.
-
Sacituzumab Govitecan in Triple Negative Breast Cancer: A Systematic Review of Clinical Trials.Cancers (Basel). 2024 Oct 27;16(21):3622. doi: 10.3390/cancers16213622. Cancers (Basel). 2024. PMID: 39518062 Free PMC article. Review.
References
-
- DeSantis C.E., Fedewa S.A., Goding Sauer A., Kramer J.L., Smith R.A., Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. - PubMed
-
- Bardia A., Mayer I.A., Vahdat L.T., et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

