Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems
- PMID: 35643078
- PMCID: PMC9308721
- DOI: 10.1016/j.neuron.2022.05.003
Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems
Abstract
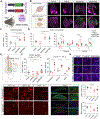
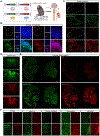
Gene therapy offers great promise in addressing neuropathologies associated with the central and peripheral nervous systems (CNS and PNS). However, genetic access remains difficult, reflecting the critical need for the development of effective and non-invasive gene delivery vectors across species. To that end, we evolved adeno-associated virus serotype 9 (AAV9) capsid in mice and validated two capsids, AAV-MaCPNS1 and AAV-MaCPNS2, across rodent species (mice and rats) and non-human primate (NHP) species (marmosets and rhesus macaques). Intravenous administration of either AAV efficiently transduced the PNS in rodents and both the PNS and CNS in NHPs. Furthermore, we used AAV-MaCPNS1 in mice to systemically deliver the following: (1) the neuronal sensor jGCaMP8s to record calcium signal dynamics in nodose ganglia and (2) the neuronal actuator DREADD to dorsal root ganglia to mediate pain. This conclusively demonstrates the translatability of these two systemic AAVs across four species and their functional utility through proof-of-concept studies in mice.
Keywords: AAV; CNS; PNS; cross-species; functional modulation; functional readout; gene therapy; non-human primate.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The California Institute of Technology has filed and licensed patent applications for some of the work described in this manuscript, with X.C., S.R.K., and V.G. listed as inventors. V.G. is a member of the Neuron advisory board and a co-founder and board member of Capsida Biotherapeutics, a fully integrated AAV engineering and gene therapy company.
Figures




References
-
- Ali Hossamaldein Gaber, Ibrahim Khalid, Elsaid Mahmoud Fawzi, Mohamed Reem Babiker, Abeidah Mahmoud I. A., Al Rawwas Azhar Othman, Elshafey Khaled, et al. 2021. “Gene Therapy for Spinal Muscular Atrophy: The Qatari Experience.” Gene Therapy, July, 1–5. 10.1038/s41434-021-00273-7. - DOI - PMC - PubMed
-
- Asencor Andoni I., Dvoryanchikov Gennady, Tsoulfas Pantelis, and Chaudhari Nirupa. 2021. “AAV-PHP.S-Mediated Delivery of Reporters to Cranial Ganglion Sensory Neurons.” 10.1101/2021.09.14.460327. - DOI
-
- Batista Ana Rita, King Oliver D., Reardon Christopher P., Davis Crystal, Shankaracharya null, Philip Vivek, Gray-Edwards Heather, et al. 2020. “Ly6a Differential Expression in Blood-Brain Barrier Is Responsible for Strain Specific Central Nervous System Transduction Profile of AAV-PHP.B.” Human Gene Therapy 31 (1–2): 90–102. 10.1089/hum.2019.186. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

