Methamphetamine induces thoracic aortic aneurysm/dissection through C/EBPβ
- PMID: 35643386
- PMCID: PMC9753351
- DOI: 10.1016/j.bbadis.2022.166447
Methamphetamine induces thoracic aortic aneurysm/dissection through C/EBPβ
Abstract
Aims: Thoracic aortic aneurysm/dissection (TAAD) is a life-threatening disease with diverse clinical manifestations. Although the association between methamphetamine (METH) and TAAD is frequently observed, the causal relationship between METH abuse and aortic aneurysm/dissection has not been established. This study was designed to determine if METH causes aortic aneurysm/dissection and delineate the underlying mechanism.
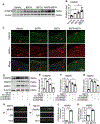
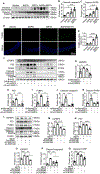
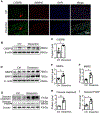
Methods and results: A new TAAD model was developed by exposing METH to SD rats pre-treated with lysyl oxidase inhibitor β-aminopropionitrile (BAPN). Combination of METH and BAPN caused thoracic aortic aneurysm/dissection in 60% of rats. BAPN+METH significantly increased the expression and activities of both matrix metalloproteinase MMP2 and MMP9, consistent with the severe elastin breakage and dissection. Mechanistically, METH increased CCAAT-enhancer binding protein β (C/EBPβ) expression by enhancing mothers against decapentaplegic homolog 3 (Smad3) and extracellular regulated protein kinase (ERK1/2) signaling. METH also promoted C/EBPβ binding to MMP2 and MMP9 promoters. Blocking C/EBPβ significantly attenuated METH+BAPN-induced TAAD and MMP2/MMP9 expression. Moreover, BAPN+METH promoted aortic medial smooth muscle cell (SMC) apoptosis through C/EBPβ-mediated IGFBP5/p53/PUMA signaling pathways. More importantly, the expression of C/EBPβ, MMP2/MMP9, and apoptosis-promoting proteins was increased in the aorta of human patients with thoracic aortic dissection, suggesting that the mechanisms identified in animal study could be relevant to human disease.
Conclusions: Our study demonstrated that METH exposure has a casual effect on TAAD. C/EBPβ mediates METH-introduced TAAD formation by causing elastin breakage, medial cell loss and degeneration. Therefore, C/EBPβ may be a potential factor for TAAD clinical diagnosis or treatment.
Keywords: Apoptosis; C/EBPβ; MMP2/9; Methamphetamine; Thoracic aortic aneurysm/dissection.
Copyright © 2022. Published by Elsevier B.V.
Figures




References
-
- Senturk T, Antal A and Gunel T. Potential function of microRNAs in thoracic aortic aneurysm and thoracic aortic dissection pathogenesis. Mol Med Rep. 2019;20:5353–5362. - PubMed
-
- El-Hamamsy I and Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–86. - PubMed
-
- LeMaire SA and Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–13. - PubMed
-
- Liu R, Lo L, Lay AJ, Zhao Y, Ting KK, Robertson EN, Sherrah AG, Jarrah S, Li H, Zhou Z, Hambly BD, Richmond DR, Jeremy RW, Bannon PG, Vadas MA and Gamble JR. ARHGAP18 Protects Against Thoracic Aortic Aneurysm Formation by Mitigating the Synthetic and Proinflammatory Smooth Muscle Cell Phenotype. Circ Res. 2017;121:512–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

