Review: Emerging roles of the signaling network of the protein kinase GCN2 in the plant stress response
- PMID: 35643606
- PMCID: PMC9197246
- DOI: 10.1016/j.plantsci.2022.111280
Review: Emerging roles of the signaling network of the protein kinase GCN2 in the plant stress response
Abstract
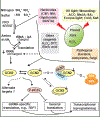
The pan-eukaryotic protein kinase GCN2 (General Control Nonderepressible2) regulates the translation of mRNAs in response to external and metabolic conditions. Although GCN2 and its substrate, translation initiation factor 2 (eIF2) α, and several partner proteins are substantially conserved in plants, this kinase has assumed novel functions in plants, including in innate immunity and retrograde signaling between the chloroplast and cytosol. How exactly some of the biochemical paradigms of the GCN2 system have diverged in the green plant lineage is only partially resolved. Specifically, conflicting data underscore and cast doubt on whether GCN2 regulates amino acid biosynthesis; also whether phosphorylation of eIF2α can in fact repress global translation or activate mRNA specific translation via upstream open reading frames; and whether GCN2 is controlled in vivo by the level of uncharged tRNA. This review examines the status of research on the eIF2α kinase, GCN2, its function in the response to xenobiotics, pathogens, and abiotic stress conditions, and its rather tenuous role in the translational control of mRNAs.
Keywords: Amino acids; Innate immunity; Reactive oxygen; Ribosome stalling; Translation initiation; tRNA.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no competing interests.
Figures



References
-
- Merchante C, Stepanova AN, Alonso JM, Translation regulation in plants: an interesting past, an exciting present and a promising future, Plant J, 90 (2017) 628–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

