AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer
- PMID: 35643725
- PMCID: PMC9148303
- DOI: 10.1038/s41598-022-12995-8
AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer
Abstract
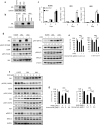
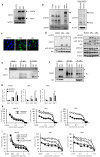
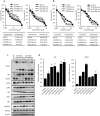
Osimertinib, a third-generation EGFR-TKI, has nowadays been applied to non-small cell lung cancer harboring activated EGFR mutation with or without T790M, but ultimately develop resistance to this drug. Here we report a novel mechanism of acquired resistance to osimertinib and the reversal of which could improve the clinical outcomes. In osimertinib-resistant lung cancer cell lines harboring T790M mutation that we established, expression of multiple EGFR family proteins and MET was markedly reduced, whereas expression of AXL, CDCP1 and SRC was augmented along with activation of AKT. Surprisingly, AXL or CDCP1 expression was induced by osimertinib in a time-dependent manner up to 3 months. Silencing of CDCP1 or AXL restored the sensitivity to osimertinib with reduced activation of SRC and AKT. Furthermore, silencing of both CDCP1 and AXL increased the sensitivity to osimertinib. Either silencing of SRC or dasatinib, a SRC family kinase (SFK) inhibitor, suppressed AKT phosphorylation and cell growth. Increased expression of AXL and CDCP1 was observed in refractory tumor samples from patients with lung cancer treated with osimertinib. Together, this study suggests that AXL/SFK/AKT and CDCP1/SFK/AKT signaling pathways play some roles in acquired osimertinib resistance of non-small cell lung cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

