Validation of donor fraction cell-free DNA with biopsy-proven cardiac allograft rejection in children and adults
- PMID: 35643770
- PMCID: PMC9617812
- DOI: 10.1016/j.jtcvs.2022.04.027
Validation of donor fraction cell-free DNA with biopsy-proven cardiac allograft rejection in children and adults
Abstract
Objectives: Donor-specific cell-free DNA shows promise as a noninvasive marker for allograft rejection, but as yet has not been validated in both adult and pediatric recipients. The study objective was to validate donor fraction cell-free DNA as a noninvasive test to assess for risk of acute cellular rejection and antibody-mediated rejection after heart transplantation in pediatric and adult recipients.
Methods: Pediatric and adult heart transplant recipients were enrolled from 7 participating sites and followed for 12 months or more with plasma samples collected immediately before all endomyocardial biopsies. Donor fraction cell-free DNA was extracted, and quantitative genotyping was performed. Blinded donor fraction cell-free DNA and clinical data were analyzed and compared with a previously determined threshold of 0.14%. Sensitivity, specificity, negative predictive value, positive predictive value, and receiver operating characteristic curves were calculated.
Results: A total of 987 samples from 144 subjects were collected. After applying predefined clinical and technical exclusions, 745 samples from 130 subjects produced 54 rejection samples associated with the composite outcome of acute cellular rejection grade 2R or greater and pathologic antibody-mediated rejection 2 or greater and 323 healthy samples. For all participants, donor fraction cell-free DNA at a threshold of 0.14% had a sensitivity of 67%, a specificity of 79%, a positive predictive value of 34%, and a negative predictive value of 94% with an area under the curve of 0.78 for detecting rejection. When analyzed independently, these results held true for both pediatric and adult cohorts at the same threshold of 0.14% (negative predictive value 92% and 95%, respectively).
Conclusions: Donor fraction cell-free DNA at a threshold of 0.14% can be used to assess for risk of rejection after heart transplantation in both pediatric and adult patients with excellent negative predictive value.
Keywords: cell-free DNA; endomyocardial biopsy; heart transplantation; pediatric heart transplantation; rejection.
Copyright © 2022 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Figures

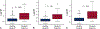
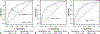
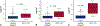
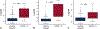

Comment in
-
Commentary: Set me free-cell-free DNA may soon provide reprieve to pediatric heart transplant recipients.J Thorac Cardiovasc Surg. 2023 Feb;165(2):469-470. doi: 10.1016/j.jtcvs.2022.05.020. Epub 2022 May 28. J Thorac Cardiovasc Surg. 2023. PMID: 35715272 No abstract available.
References
-
- Khush KK, Cherikh WS, Chambers DC et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1056–1066. - PMC - PubMed
-
- Rossano JW, Singh TP, Cherikh WS et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report - 2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1028–1041. - PMC - PubMed
-
- Angelini A, Andersen CB, Bartoloni G et al. A web-based pilot study of inter-pathologist reproducibility using the ISHLT 2004 working formulation for biopsy diagnosis of cardiac allograft rejection: the European experience. J Heart Lung Transplant 2011;30:1214–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

