Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial
- PMID: 35643868
- PMCID: PMC9252917
- DOI: 10.1038/s41375-022-01589-y
Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial
Abstract
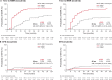
This analysis from the multicenter, open-label, phase 3 BFORE trial reports efficacy and safety of bosutinib in patients with newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML) after five years' follow-up. Patients were randomized to 400-mg once-daily bosutinib (n = 268) or imatinib (n = 268; three untreated). At study completion, 59.7% of bosutinib- and 58.1% of imatinib-treated patients remained on study treatment. Median duration of treatment and time on study was 55 months in both groups. Cumulative major molecular response (MMR) rate by 5 years was higher with bosutinib versus imatinib (73.9% vs. 64.6%; odds ratio, 1.57 [95% CI, 1.08-2.28]), as were cumulative MR4 (58.2% vs. 48.1%; 1.50 [1.07-2.12]) and MR4.5 (47.4% vs. 36.6%; 1.57 [1.11-2.22]) rates. Superior MR with bosutinib versus imatinib was consistent across Sokal risk groups, with greatest benefit seen in patients with high risk. Treatment-emergent adverse events (TEAEs) were consistent with 12-month data. After 5 years of follow-up there was an increase in the incidence of cardiac, effusion, renal, and vascular TEAEs in bosutinib- and imatinib-treated patients, but overall, no new safety signals were identified. These final results support 400-mg once-daily bosutinib as standard-of-care in patients with newly diagnosed CP CML.This trial was registered at www.clinicaltrials.gov as #NCT02130557.
© 2022. The Author(s).
Conflict of interest statement
THB served as a consultant for Janssen, Merck, Novartis, and Pfizer; received research funding from Novartis and Pfizer; received honorarium from Pfizer. JEC served as a consultant for Amphivena Therapeutics, Astellas Pharma, Bio-Path Holdings Inc, BiolineRx, Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Novartis, Pfizer, and Takeda; received research funding from Astellas Pharma, Bristol Myers Squibb, Daiichi Sankyo, Immunogen, Jazz Pharmaceuticals, Merus, Novartis, Pfizer, Sun Pharma, Takeda, Tolero Pharmaceuticals, and Tovagene. DM served as a consultant for Bristol Myers Squibb, Incyte, Pfizer, and Novartis; received research funding from Pfizer. CG-P served as a consultant for Bristol Myers Squibb; received research funding and honorarium from Pfizer. REC served as a consultant for AbbVie, Bristol Myers Squibb, Jazz Pharmaceuticals, and Novartis; received research funding from Bristol Myers Squibb, Novartis, and Pfizer; received honorarium from Ariad/Incyte, Bristol Myers Squibb, Novartis, and Pfizer. PleC received honorarium from Incyte, Novartis, and Pfizer; received research funding from Pfizer. VG-G served as a consultant for Bristol Myers Squibb, Incyte, Novartis, and Pfizer; received research funding from Pfizer. CC received research funding from Bristol Myers Squibb and Pfizer; received honorarium from Bristol Myers Squibb, Korea Otsuka Pharmaceuticals, and Novartis. VK received honorarium from Ariad, Incyte, Novartis, Pfizer, and Xcenda; received research funding from Pfizer. JHL served as a consultant for Bristol Myers Squibb, Novartis, Pfizer, and Takeda; received research funding from Bristol Myers Squibb, Novartis, Pfizer, and Takeda; received honorarium from Bristol Myers Squibb, Pfizer, and Takeda. PR served as a consultant for Bristol Myers Squibb, Incyte, Novartis, Pfizer, and Takeda; received research funding from Pfizer. MJM served as a consultant for Bristol Myers Squibb, Novartis, Takeda, and Pfizer; received research funding from Bristol Myers Squibb, Novartis, Pfizer, and Sun Pharma/SPARC. AH received research funding from Bristol Myers Squibb, Incyte, Novartis, and Pfizer; received honoraria from Bristol Myers Squibb, Incyte, Novartis, and Pfizer. RHM served as a consultant for Incyte and Pfizer; received research funding from Pfizer. EL, SP, AY, and AV are employees of Pfizer and own stocks in Pfizer. MWD served as a consultant for Ariad, Blueprint Medicine, Bristol Myers Squibb, Galena Biopharma, Incyte, Novartis, and Pfizer; received research funding from Bristol Myers Squibb, Celgene, Gilead Sciences, Incyte, Novartis, and Pfizer; received honorarium from Ariad, Blueprint Medicine, Bristol Myers Squibb, Galena Biopharma, Incyte, Novartis, and Pfizer.
Figures


References
-
- Pfizer Inc. Bosulif® (bosutinib) prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=884. Accessed 9 Aug, 2021.
-
- Kantarjian HM, Mamolo CM, Gambacorti-Passerini C, Cortes JE, Brummendorf TH, Su Y, et al. Long-term patient-reported outcomes from an open-label safety and efficacy study of bosutinib in Philadelphia chromosome-positive chronic myeloid leukemia patients resistant or intolerant to prior therapy. Cancer. 2018;124:587–95.. doi: 10.1002/cncr.31082. - DOI - PMC - PubMed
-
- Gambacorti-Passerini C, Cortes JE, Lipton JH, Kantarjian HM, Kim DW, Schafhausen P, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298–307.. doi: 10.3324/haematol.2017.171249. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

