Influence of SARS-CoV-2 inactivation by different chemical reagents on the humoral response evaluated in a murine model
- PMID: 35644072
- PMCID: PMC9125173
- DOI: 10.1016/j.molimm.2022.05.012
Influence of SARS-CoV-2 inactivation by different chemical reagents on the humoral response evaluated in a murine model
Abstract
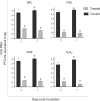
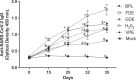
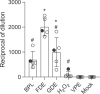
Viral inactivation for antibody induction purposes, among other applications, should ensure biosafety, completely avoiding the risk of infectivity, and preserving viral immunogenicity. β-propiolactone (BPL) is one of the most used reagents for viral inactivation, despite its high toxicity and recent difficulties related to importation, experienced in Brazil during the SARS-CoV-2 pandemic. In this context, the main objectives of this work were to test different inactivation procedures for SARS-CoV-2 and to evaluate the induction of neutralizing antibodies in mice immunized with antigenic preparations obtained after viral treatment with formaldehyde (FDE), glutaraldehyde (GDE), peroxide hydrogen (H2O2), as well as with viral proteins extract (VPE), in parallel with BPL. Verification of viral inactivation was performed by subsequent incubations of the inactivated virus in Vero cells, followed by cytopathic effect and lysis plaques observation, as well as by quantification of RNA load using reverse transcription-quantitative real time polymerase chain reaction. Once viral inactivation was confirmed, cell culture supernatants were concentrated and purified. In addition, an aliquot inactivated by BPL was also subjected to viral protein extraction (VPE). The different antigens were prepared using a previously developed microemulsion as adjuvant, and were administered in a four-dose immunization protocol. Antibody production was comparatively evaluated by ELISA and Plaque Reduction Neutralization Tests (PRNT). All immunogens evaluated showed some level of IgG anti-SARS-CoV-2 antibodies in the ELISA assay, with the highest levels presented by the group immunized with FDE-inactivated viral antigen. In the PRNT results, except for VPE-antigen, all other immunogens evaluated induced some level of neutralizing anti-SARS-CoV-2 antibodies, and the FDE-antigen stood out again with the most expressive values. Taken together, the present work shows that FDE can be an efficient and affordable alternative to BPL for the production of inactivated SARS-CoV-2 viral antigen.
Keywords: ELISA; Inactivated virus; PRNT(50); RT-qPCR; SARS-CoV-2.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

