Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials
- PMID: 35644168
- PMCID: PMC9135375
- DOI: 10.1016/S2213-2600(22)00131-X
Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials
Abstract
Background: All currently available SARS-CoV-2 vaccines are administered by intramuscular injection. We aimed to evaluate the safety and immunogenicity of a live-attenuated influenza virus vector-based SARS-CoV-2 vaccine (dNS1-RBD) administered by intranasal spray in healthy adults.
Methods: We did double-blind, randomised, placebo-controlled phase 1 and 2 trials, followed by a phase 2 extension trial, at a single centre in Jiangsu, China. Healthy adults (≥18 years) who had negative serum or fingertip blood total antibody tests for SARS-CoV-2 (in phases 1 and 2), with no prevalent SARS-CoV-2 infection or history of infection and no SARS-CoV-2 vaccination history (in all three trials reported here), were enrolled. Participants were randomly allocated (4:1 in phase 1, 2:1 in phase 2, and 1:1 in the extension trial) to receive two intranasal doses of the dNS1-RBD vaccine or placebo on days 0 and 14 or, for half of the participants in phase 2, on days 0 and 21. To avoid cross-contamination during administration, vaccine and placebo recipients were vaccinated in separate rooms in the extension trial. The phase 1 primary outcome was safety (adverse events recorded on days 0-44; serious adverse events recorded from day 0 until 12 months after the second dose). In the phase 2 and extension trials, the primary immunogenicity outcomes were SARS-CoV-2-specific T-cell response in peripheral blood (measured by IFN-γ ELISpot), proportion of participants with positive conversion for SARS-CoV-2 receptor-binding domain (RBD)-specific IgG and secretory IgA (s-IgA) antibodies, and concentration of SARS-CoV-2 RBD IgG in serum and SARS-CoV-2 RBD s-IgA in the nasopharynx (measured by ELISA) at 1 month after the second dose in the per-protocol set for immunogenicity. χ2 test and Fisher's exact test were used to analyse categorical data, and t test and Wilcoxon rank sum test to compare the measurement data between groups. These trials were registered with the Chinese Clinical Trial Registry (ChiCTR2000037782, ChiCTR2000039715, and ChiCTR2100048316).
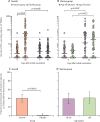
Findings: Between Sept 1, 2020, and July 4, 2021, 63, 724, and 297 participants without a history of SARS-CoV-2 vaccination were enrolled in the phase 1, phase 2, and extension trials, respectively. At least one adverse reaction after vaccination was reported in 133 (19%) of 684 participants in the vaccine groups. Most adverse reactions were mild. No vaccine-related serious adverse event was noted. Specific T-cell immune responses were observed in 211 (46% [95% CI 42-51]) of 455 vaccine recipients in the phase 2 trial, and in 48 (40% [31-49]) of 120 vaccine recipients compared with one (1% [0-5]) of 111 placebo recipients (p<0·0001) in the extension trial. Seroconversion for RBD-specific IgG was observed in 48 (10% [95% CI 8-13]) of 466 vaccine recipients in the phase 2 trial (geometric mean titre [GMT] 3·8 [95% CI 3·4-4·3] in responders), and in 31 (22% [15-29]) of 143 vaccine recipients (GMT 4·4 [3·3-5·8]) and zero (0% [0-2]) of 147 placebo recipients (p<0·0001) in the extension trial. 57 (12% [95% CI 9-16]) of 466 vaccine recipients had positive conversion for RBD-specific s-IgA (GMT 3·8 [95% CI 3·5-4·1] in responders) in the phase 2 trial, as did 18 (13% [8-19]) of 143 vaccine recipients (GMT 5·2 [4·0-6·8]) and zero (0% [0-2]) of 147 placebo recipients (p<0·0001) in the extension trial.
Interpretation: dNS1-RBD was well tolerated in adults. Weak T-cell immunity in peripheral blood, as well as weak humoral and mucosal immune responses against SARS-CoV-2, were detected in vaccine recipients. Further studies are warranted to verify the safety and efficacy of intranasal vaccines as a potential supplement to current intramuscular SARS-CoV-2 vaccine pools. Steps should be taken in future studies to reduce the potential for cross-contamination caused by the vaccine strain aerosol during administration.
Funding: National Key Research and Development Program of China, National Science, Fujian Provincial Science, CAMS Innovation Fund for Medical Sciences, and Beijing Wantai Biological Pharmacy Enterprise.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests QS was an employee of Beijing Wantai Biological Pharmacy Enterprise during the conduct of the study. JH and XY are employees of and have stock options in Beijing Wantai Biological Pharmacy Enterprise. All other authors declare no competing interests.
Figures



References
-
- WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
-
- Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–2143. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

